Sterile polymerized covering dressing for wound surface
A wound repair and amorphous technology, applied in bandages, absorbent pads, medical science and other directions, can solve the problems of cumbersome preparation process, unsuitable for industrial production, etc., and achieve the effect of avoiding cross infection, reducing temperature, and avoiding air permeability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
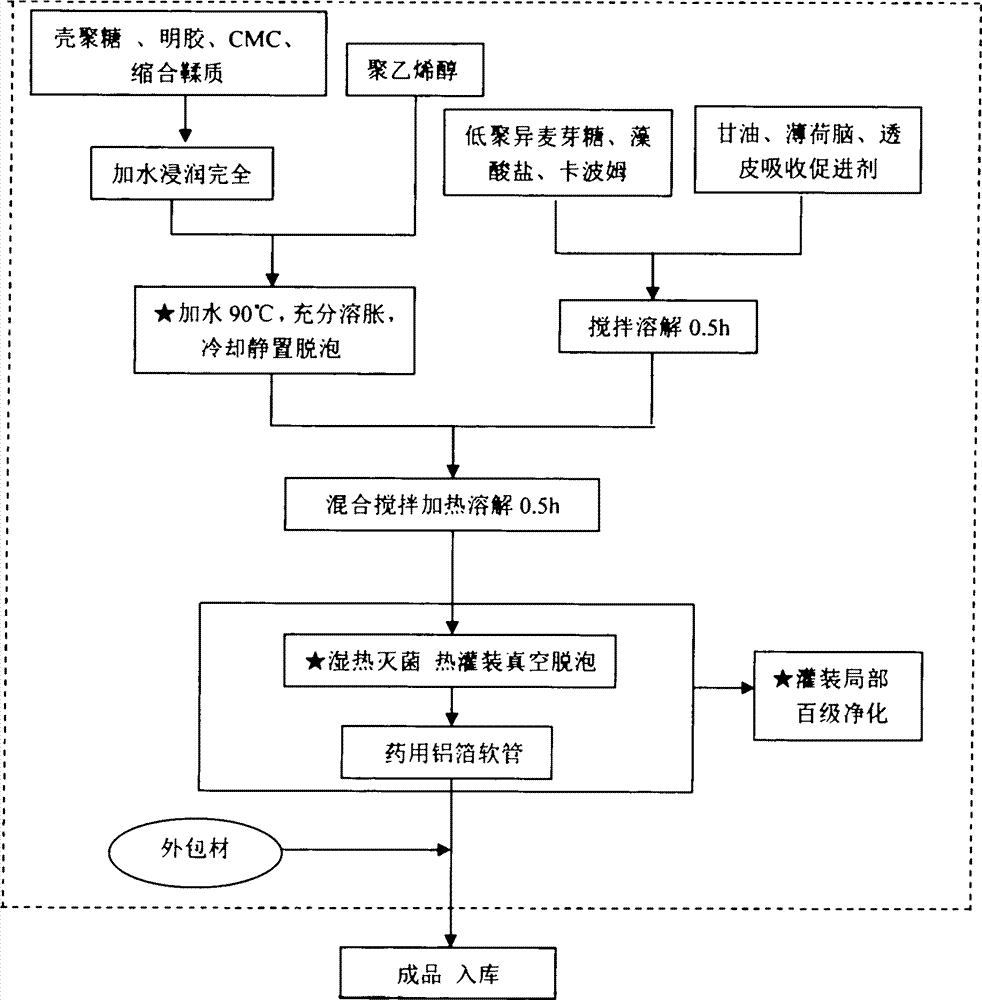
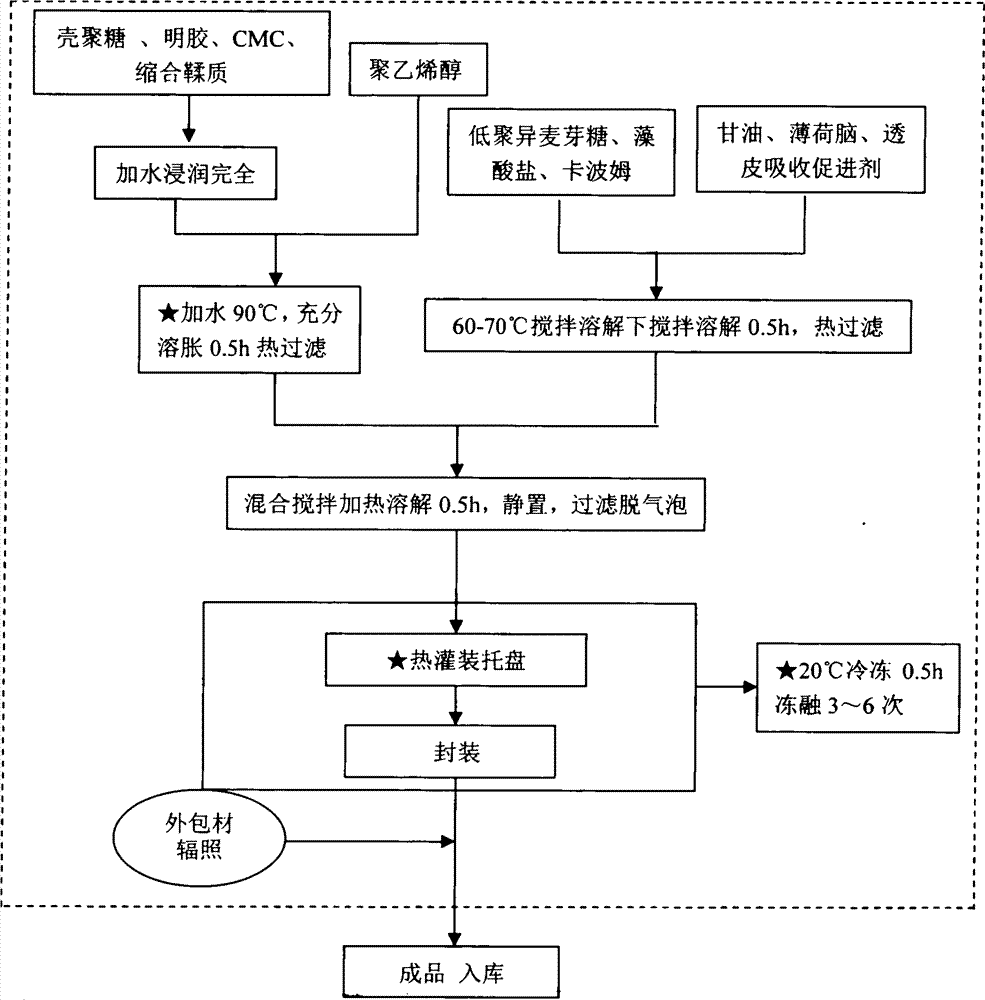
[0117] Chitosan 10g, polyvinyl alcohol 30g, alginate 5g, isomaltooligosaccharide 10g, Carbopol 940 2g, gelatin 2g, carboxymethylcellulose sodium 5g, glycerin 10g, menthol 0.01g, condensed tannins 15g. Transdermal absorption accelerator synthetic borneol 5g, appearance regulator theaflavin 0.02g. For the preparation method, refer to the steps and methods for the preparation of the semi-solid amorphous dressing.
[0118] Animal model experiment results:
[0119]
[0120] Clinical Trial Results:
[0121]
[0122] Clinical trial conclusion: There were no abnormal changes in the two routine tests, and no severe allergic adverse reactions in the whole body or treatment site. The patient has strong acceptability of dressing change, and the pain is significantly reduced. The acceptability of doctors and patients has been verified in this clinical trial. Medical moist burn wound dressings can accelerate wound healing, relieve pain, and increase the acceptability of patients' ...
Embodiment 2
[0124] Chitosan 15g, polyvinyl alcohol 40g, high viscosity alginate 6g, isomaltooligosaccharide 12g, Carbopol 9403.5g, gelatin 3g, carboxymethylcellulose sodium 7g, glycerin 15g, menthol 0.08g, condensation Tannin 30g. The appearance regulator copper chlorophyllin sodium salt 0.02g. For the preparation method, refer to the steps and methods for the preparation of the semi-solid amorphous dressing.
[0125] Animal model experiment results:
[0126]
Embodiment 3
[0128] Chitosan 18g, polyvinyl alcohol 50g, alginate 14g, isomaltooligosaccharide 14g, Carbopol 940 4.5g, gelatin 4.5g, sodium carboxymethylcellulose 8g, glycerin 18g, menthol 0.1g. Transdermal absorption enhancer propylene glycol 10g, appearance regulator theaflavin 0.02g. For the preparation method, refer to the steps and methods for the preparation of the semi-solid amorphous dressing.
[0129] Animal model experiment results:
[0130]
[0131]
[0132] Clinical Trial Results:
[0133]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com