Polymorphs of alogliptin benzoate
A benzoic acid, form of technology, applied in the field of new form of alogliptin benzoate, can solve unmet problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: General preparation method of alogliptin benzoate polymorph
[0075] 1. Reagents
[0076] Acetonitrile, HPLC grade, Sigma, lot number MKBC1316
[0077] Ethanol, AR, SCRC, lot number T10000418
[0078] DMSO, HPLC grade, Sigma, lot number 05737BH
[0079] Dichloromethane, AR, SCRC, Lot No. 80047318
[0080] Methanol, AR, SCRC, Lot No. 80080418
[0081] Ethanol Acetate (Ethanol Acetate), AR, Yixing Secondary Chemical Company (YixingSecondary Chemical Company), batch number 090607
[0082] MIBK, AR, SCRC, batch number T20080411
[0083] Isopropanol, AR, Sinopharm Chemical Reagent Co.Ltd, batch number T20090813
[0084] Acetone, AR, Sinopharm Chemical Reagent Co.Ltd, batch number 10000418
[0085] Toluene, AR, SCRC, batch number T20090603
[0086] tert-Butyl methyl ether, HPLC grade, Fluka, lot number 1359496
[0087]THF, AR, Yixing Secondary Chemical Company (Yixing Secondary Chemical), batch number 090901
[0088] n-Butanol, AR, SCRC, batch number...
Embodiment 2
[0138] Example 2: Amorphous Alogliptin Benzoate Form I (Method I)
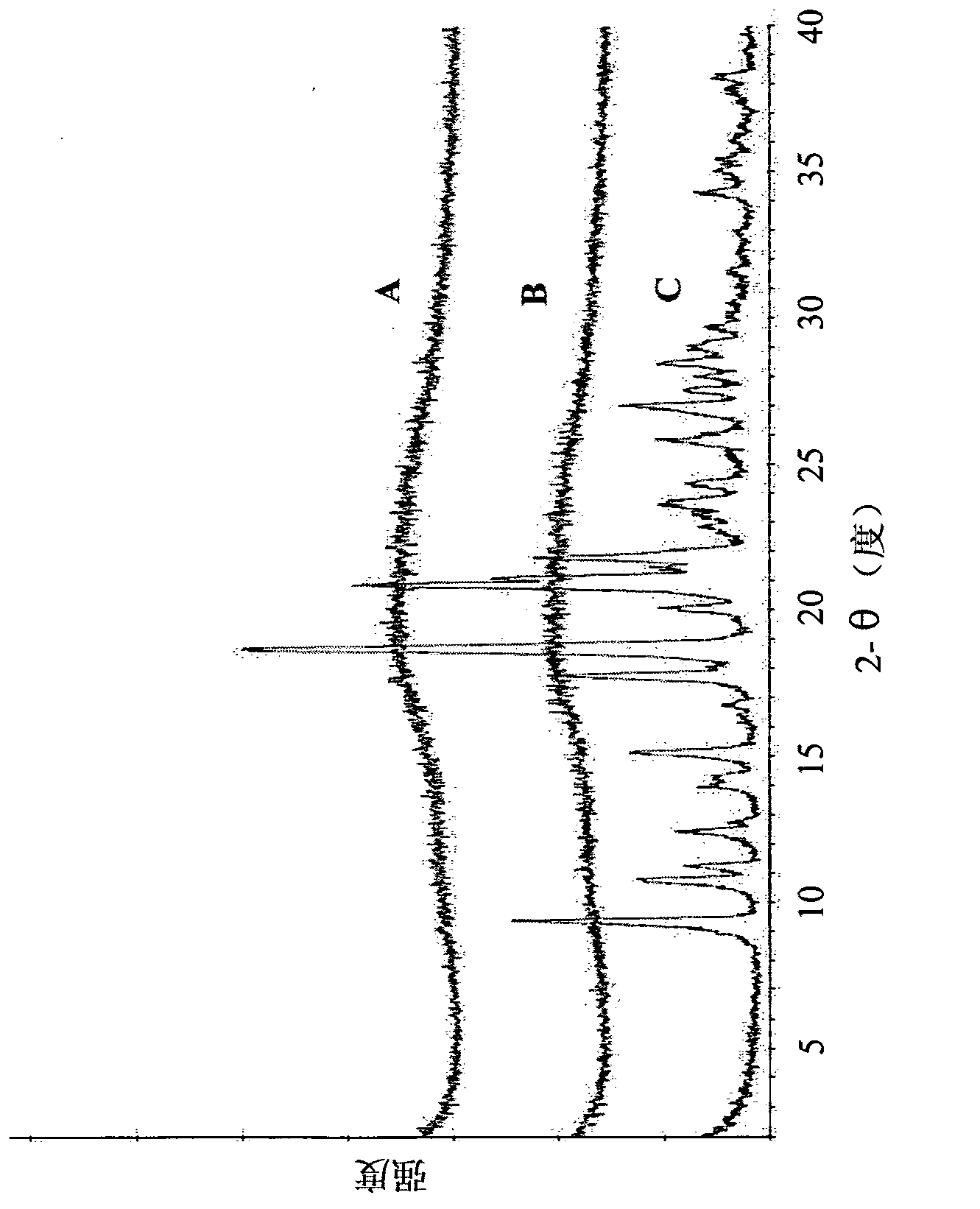
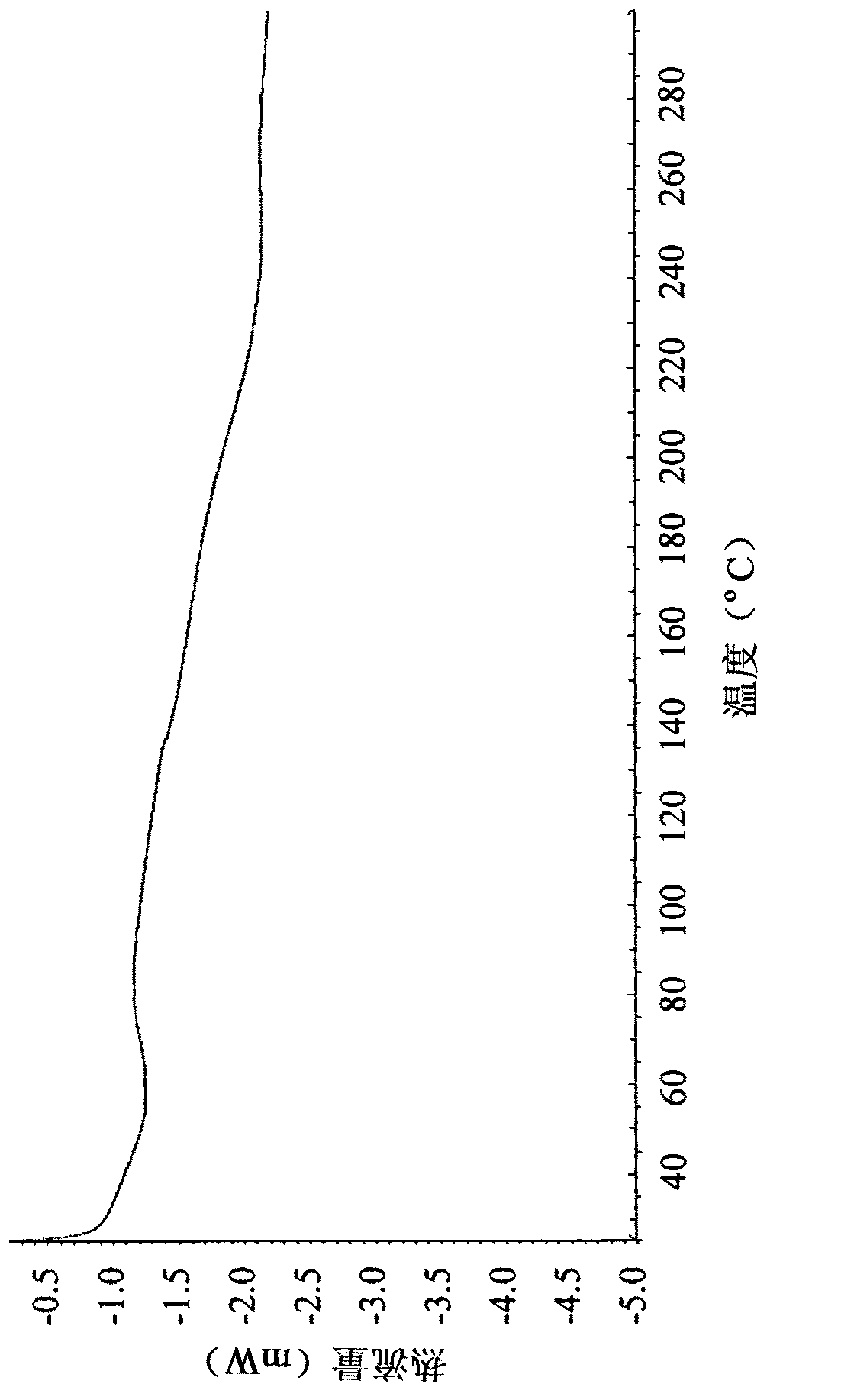
[0139] Implement general method I. Thus, the alogliptin API was heated to 200° C., followed by rapid cooling (quenching) or slow cooling. This new polymorphic form exhibits an amorphous powder ( figure 1 , panels A and B) characteristic broad X-ray diffraction peaks between about 10 and about 35 [2θ°]. The amorphous phase is stable even after heating to 300°C. figure 2 A characteristic DSC curve is shown. The DSC curve of the amorphous alogliptin benzoate Form I of the present invention is significantly different from the DSC curve of the amorphous alogliptin benzoate disclosed in WO2007 / 035372. For example, the amorphous alogliptin benzoate form I of the present invention exhibits a relatively smooth DSC curve with no exothermic peak at 132°C and no endothermic peak at 183°C, unlike the amorphous alogliptin benzoate in WO 2007 / 035372 Form 1 is the opposite. According to WO 2007 / 035372, recrystallizat...
Embodiment 3
[0141] Example 3: Amorphous Alogliptin Benzoate Form II (Method II)
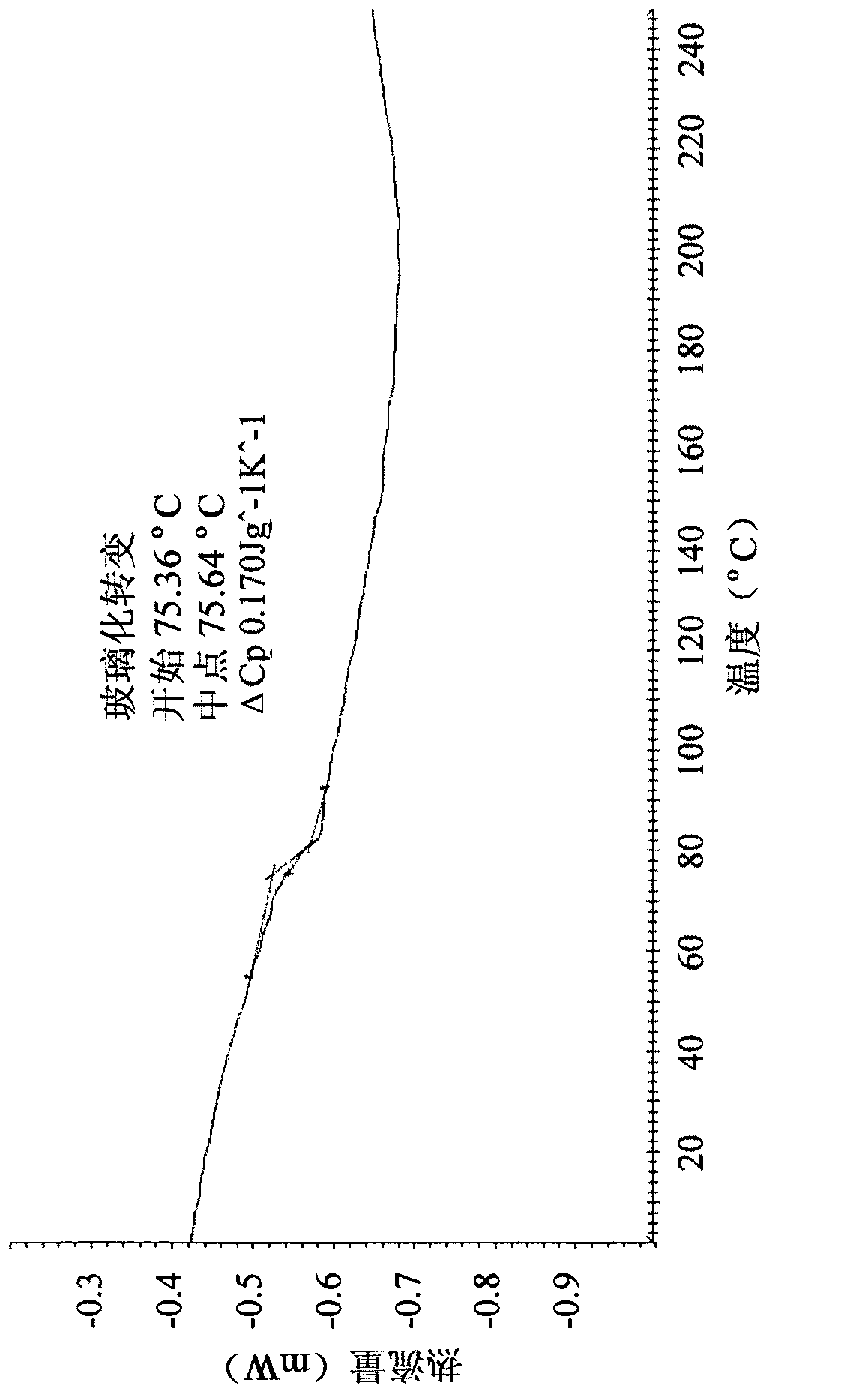
[0142] Implement general method II. Thus, alogliptin API was dissolved in EtOH at room temperature until a saturated solution of alogliptin was obtained. The solvent was then removed by rotary evaporation below 50 °C. This new polymorphic form exhibits an amorphous powder ( Figure 7 , Panel C) characteristic broad X-ray diffraction peaks between about 10 and about 35 [2θ°]. Figure 8 A characteristic DSC curve is shown with an exothermic peak at about 128°C followed by an endothermic peak at 182°C. After DSC measurement, this amorphous form crystallized into alogliptin API. Amorphous Form II of the present invention is further characterized by modulating the DSC in order to determine its glass transition temperature ( Figure 9 ). The glass transition temperature is between about 68°C and about 73°C (variation between batches is mainly due to the effect of residual solvent). Figure 10 Characterist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Variable temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com