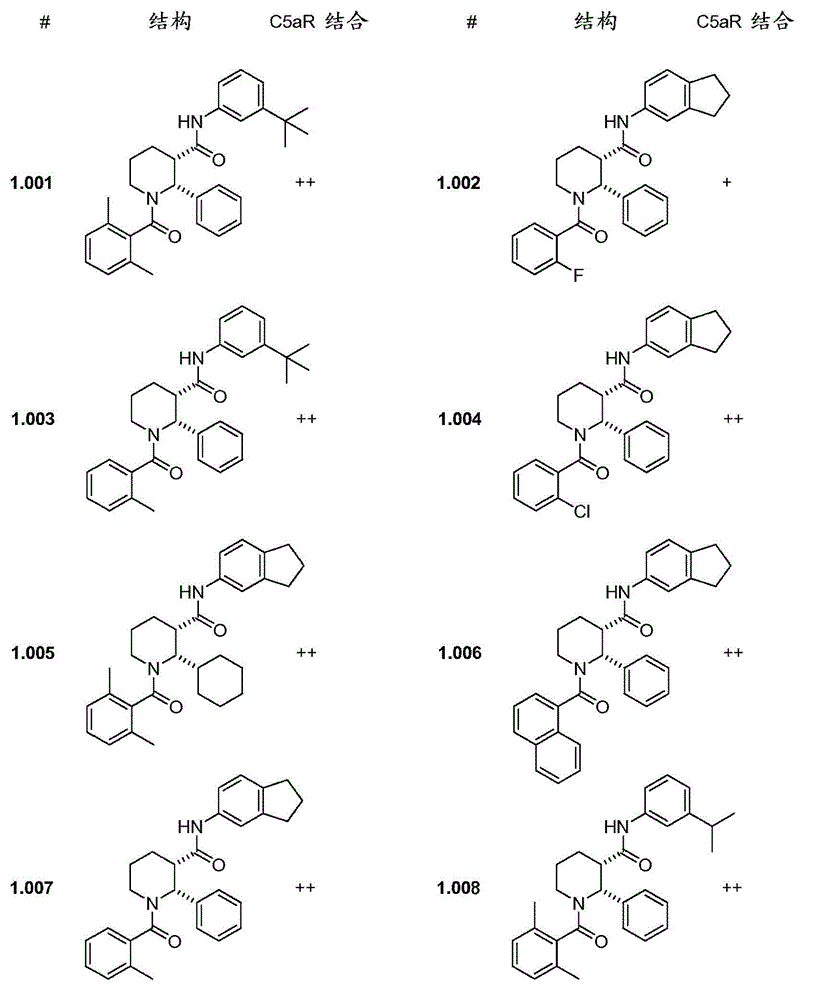
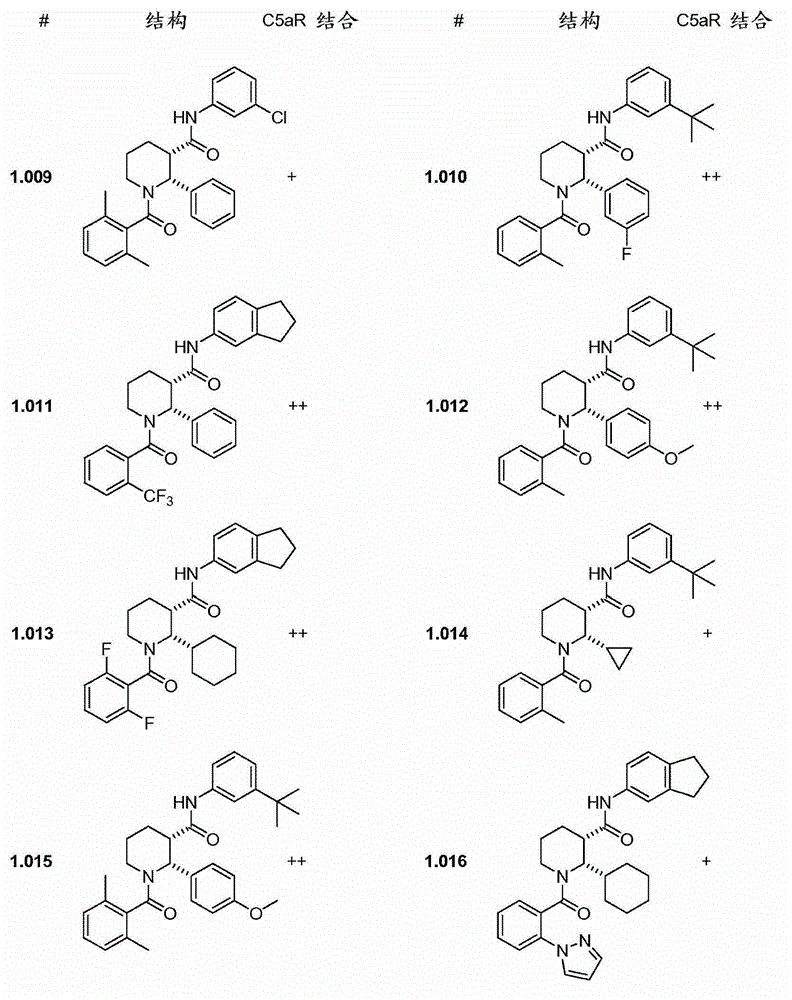
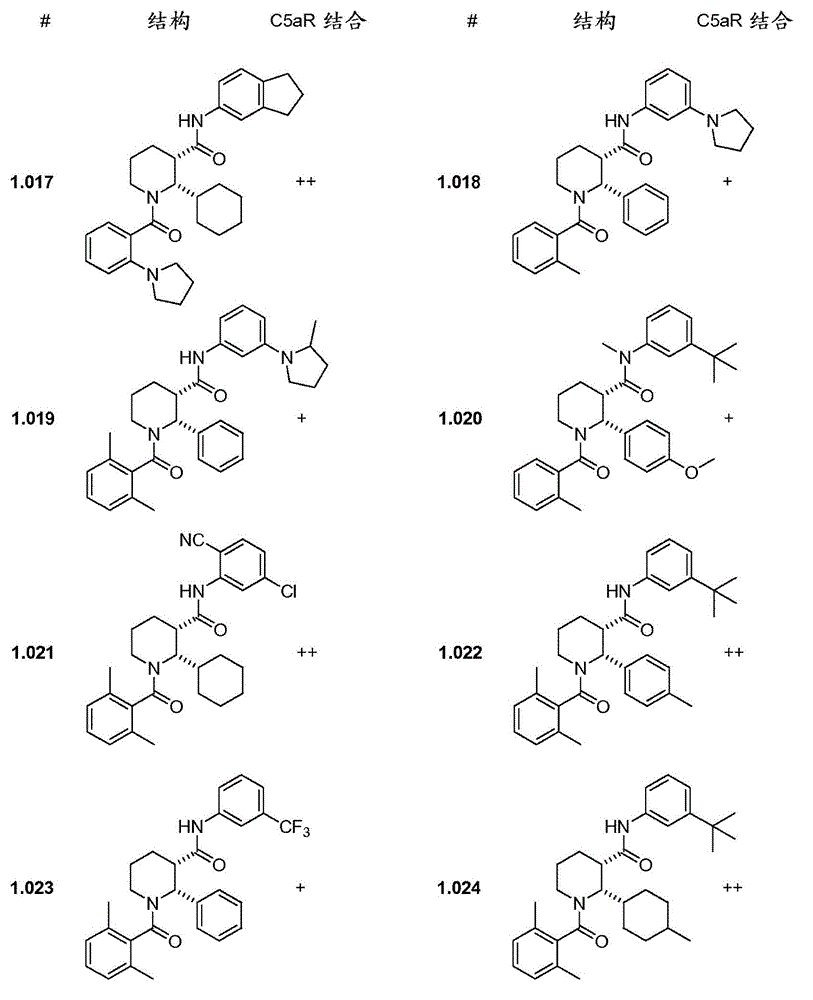
C5ar antagonists
A technology selected from and compounds, applied in the direction of anti-inflammatory agents, antibacterial drugs, anti-tumor drugs, etc., can solve the problem of reducing pDC infiltration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] Compound preparation
[0102] Those skilled in the art will recognize that there are a variety of methods that can be used to synthesize the molecules shown in the claims. In general, the method available for the synthesis of the compounds shown in the claims consists of four parts, which can be performed in any order: formation of the piperidine ring, installation of two amide bonds, and installation and / or modification of C 1 、C 2 and C 3 functional groups on the .
[0103] Several methods of preparing the claimed compounds are illustrated below (Schemes 1-6).
[0104] Reaction 1
[0105] Reaction 2
[0106] Reaction 3
[0107] Reaction 4
[0108] Schemes 1-4 show some methods for forming piperidine rings. Coupling at the 2-position of the pyridine ring can be achieved by transition metal-mediated coupling as shown in Schemes 1-2, or by metal-catalyzed addition of organometallic species such as zincate or magnesium salts (reaction Equation 3) to achie...
Embodiment 1
[0167] Synthesis of cis-1-(2-fluoro-6-methylbenzoyl)-2-phenylpiperidine-3-carboxylic acid (3-trifluoromethylphenyl)amide
[0168]
[0169] a) Pd(PPh 3 ) 4 (3.0g, 2.6mmol) was added to 2-chloro-3-carboxyethylpyridine (25g, 134.7mmol), phenylboronic acid (21.04g, 172.6mmol) and K 2 CO 3 (55.1 g, 399 mmol) in a solution in 1,4-dioxane (200 mL) and water (200 mL). The reaction mixture was heated at 100°C for 2 hours. The solution was then cooled to room temperature and dioxane was removed under reduced pressure. The resulting aqueous layer was extracted with ethyl acetate, and the combined organic layers were dried (Na 2 SO 4 ), filtered through celite, and concentrated under reduced pressure. By flash chromatography (SiO 2 , 10-100% EtOAc / hexanes) to obtain 2-phenylpyridine derivatives (yield 91%, 27.98 g). LC-MS R t (Retention time): 2.45 minutes, MS: (ES) m / z 228 (M+H + ).
[0170] b) PtO 2 (800 mg, 3.52 mmol) was added to a solution of ethyl 2-phenyl-nicotinate...
Embodiment 2
[0176] Synthesis of N-(3-tert-butylphenyl)-1-(5-chloro-3-methylpicolyl)-2-phenylpiperidine-3-carboxamide
[0177]
[0178] a) 2-Chloronicotinoyl chloride (1.05 equiv) dissolved in anhydrous dichloromethane (0.5M) was added to 3-tert-butylaniline (1 equiv) and 2M K over 30 min at 0 °C 2 CO 3 aqueous solution (2.2 equiv) in anhydrous dichloromethane (0.5M) and the reaction mixture was stirred at room temperature for an additional 1.5 hours. The layers were separated and the aqueous layer was extracted with dichloromethane. The combined organic layers were washed with brine, dried (MgSO 4 ), filtered and concentrated to afford the desired amide as a foamy solid which was used in the next step without further purification. MS: (ES) m / z289.1 (M+H + ).
[0179] b) Pd(PPh 3 ) 4 (2-5mol%) to the above pyridine amide (1 eq), phenylboronic acid (1.4 eq) and 2M K 2 CO 3 aqueous solution (2.4 eq) in toluene (0.7 M) and heated the reaction mixture at 100° C. overnight (about 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com