L-asparaginase variant with increased activity
An asparaginase and variant technology, applied in the field of medical bioengineering, can solve the problems of aggravating the patient's economic burden, pyrogen reaction, high price and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
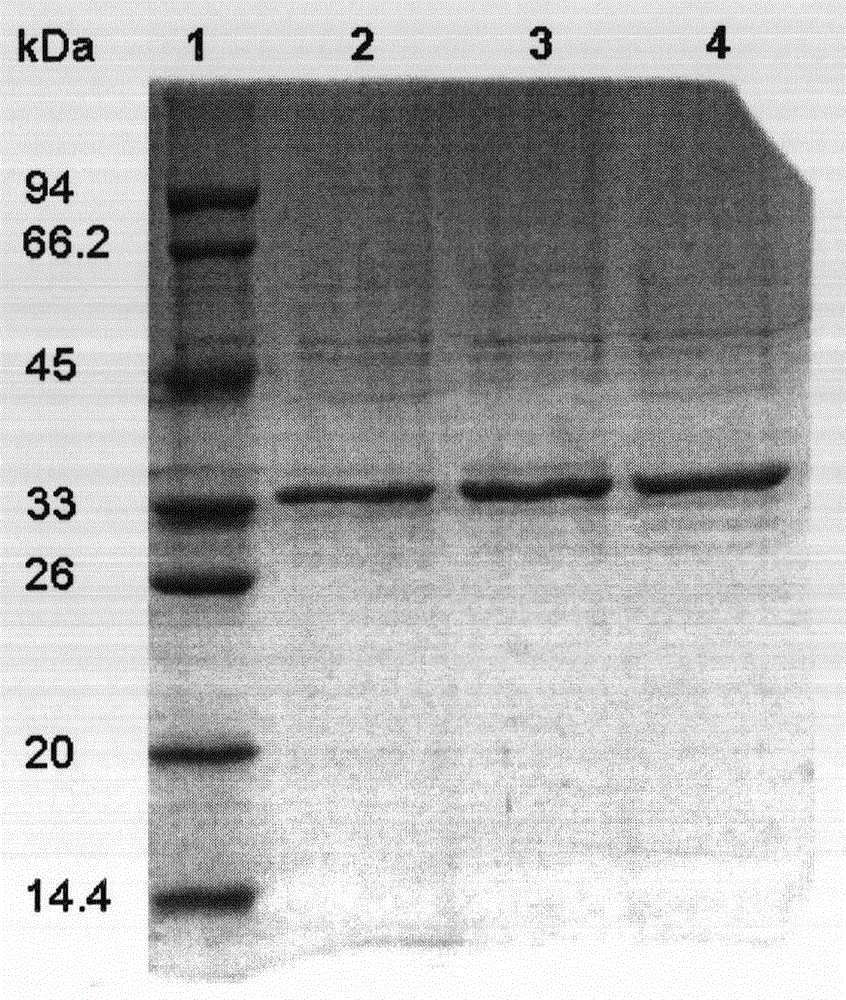
Image
Examples
Embodiment 1
[0044] Embodiment 1: Cloning of Escherichia coli L-ansB gene
[0045] According to the L-ansB nucleotide sequence published by NCBI, the upstream primer mansB-001 (5'-CG GAATTC ATGGAGTTTTTCAAAAAGACG-3') (SEQ ID NO: 1) and downstream primer mansB-002 (5'-CG GGATCC TTAGTACTGATTGAAGATCTG-3') (SEQ ID NO: 2), where the underlines show the additionally added EcoRI and BamHI restriction sites, and two additional protective bases are added at the 5' end of the primer.
[0046] PCR reaction was carried out with E. coli DH5α genome as template. The reaction conditions were: pre-denaturation at 95°C for 5 min, followed by 30 cycles of reaction according to the following parameters: denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 90 s, and finally extension at 72°C for 10 min.
[0047] After the PCR product was subjected to 1% low-melting point agarose gel electrophoresis, the gel piece containing the target fragment was cut out and placed in a 1.5mL E...
Embodiment 2
[0053] Embodiment 2: Construction of recombinant expression construct pBV-ansB
[0054] Carry out EcoRI and BamHI (Bao Biological Engineering (Dalian) Co., Ltd.) double enzyme digestion reaction on the sequencing construct pMD18T-ansB in Example 1 to recover the L-ansB target gene fragment:
[0055]
[0056] After mixing, the enzyme digestion reaction solution was placed at 37° C. for overnight incubation, and then purified and recovered by the method in Example 1. The pBV220 expression vector was digested with EcoRI and BamHI in the same manner, and the linear pBV220 expression vector fragment was purified and recovered.
[0057] The purified L-ansB target gene fragment and the linear pBV220 expression vector fragment were ligated according to the following system:
[0058]
[0059]
[0060] The ligation reaction solution after mixing was placed at 25°C for 30 minutes, and then used at 4°C for later use or directly for transformation reaction.
[0061] Add 10 μL of...
Embodiment 3
[0062] Example 3: Error-prone PCR random mutation of L-ansB gene
[0063] Prepare the error-prone PCR reaction solution according to the following system:
[0064] 10 μL of 10×PCR buffer
[0065] ·50×dNTPs mixture 2μL
[0066] · 10mM dCTP 2μL
[0067] · 10mM dTTP 2μL
[0068] 10×MgCl 2 10μL
[0069] · MnCl 2 5μL
[0070] ·Upstream primer mansB-001 2μL
[0071] ·Downstream primer mansB-002 2μL
[0072] ·Template pBV-ansB 10~100ng
[0073] · rTaq DNA polymerase 1μL (5U)
[0074] Add double distilled water to 100μL
[0075] in:
[0076] 10×PCR buffer: 20mM MgCl2, 200mM KCl, 50mM Tris-HCl, pH8.3;
[0077] 50×dNTPs mixture: containing 10mM each of dATP, dGTP, dTTP, and dCTP;
[0078] 10×MgCl 2 Solution: 70mM, dissolved in sterile water;
[0079] MnCl 2 Solution: 1mM, dissolved in sterile water;
[0080] rTaq DNA polymerase: purchased from Treasure Bioengineering (Dalian) Co., Ltd., catalog number DR001.
[0081] The error-prone PCR rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com