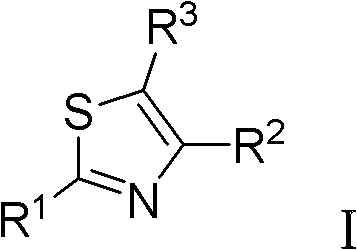
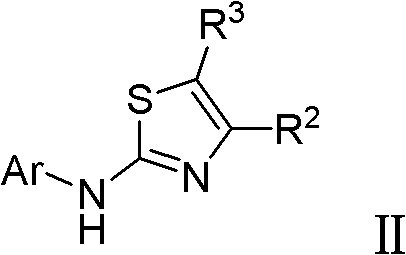
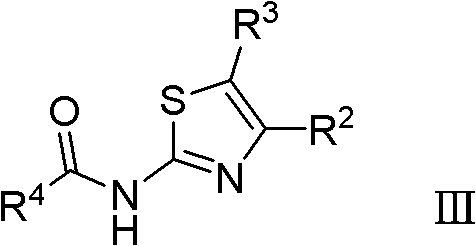
Thiazole derivative acting as DHODH inhibitor and its application
A compound, C1-C6 technology, applied in the field of medicinal chemistry and pharmacotherapeutics, can solve the problems of mucositis side effects, narrow therapeutic window, and restrictions on wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the synthetic general method of compound 1-18:
[0063]
[0064] Dissolve ethyl chloroacetoacetate (9.52g, 57.84mmol) in 100mL ethanol, add thioacetamide (4.8g, 63.9mmol) under stirring condition, raise the temperature to reflux temperature, stir and reflux for 2h, and wait for the reaction to be completed. The solvent was removed from the reaction solution under reduced pressure to obtain 9.80 g of an orange-pink solid with a yield of 91.1%, which was directly used in the next reaction.
[0065] Weigh LiOH·H 2 O (1.25g, 29.7mmol) was dissolved in 50mL of water, and the ester (2.5g, 13.5mmol) synthesized by one-step reaction was added under stirring conditions, and the temperature was raised to 90°C. As the reaction progressed, the solid gradually dissolved, and the reaction was stopped after 1h. The pH of the reaction solution was adjusted to neutral to slightly acidic with dilute hydrochloric acid, and a large amount of solids precipitated out. After...
Embodiment 2
[0140] Embodiment 2: the synthetic general method of compound 19-45:
[0141] Synthesis of Thiourea
[0142]
[0143] Dissolve arylamine (8mmol, 1eq) in 24mL of acetone, add the weighed triethylenediamine (24mmol, 3eq) under stirring conditions, then add 20mL of carbon disulfide dropwise, a large amount of solid appears, continue to stir at room temperature for 24h, filter the reaction with suction solution, the filter cake was washed with petroleum ether, and after drying, the filter cake was dissolved in 50 mL of chloroform, and BTC (2.7 mmol, 0.33 eq) was weighed and dissolved in 30 mL of chloroform, added dropwise to the reaction solution within 1 h, and stirred at room temperature. solution overnight. After the reaction, the reaction solution was suction filtered, the filter cake was washed with dichloromethane, the obtained filtrate was directly added to silica gel and spin-dried, and then dry-loaded (PE eluted).
[0144] Dissolve NCS in a small amount of dichlorome...
Embodiment 3
[0240] Embodiment 3: the synthesis general method of compound 46-63:
[0241]
[0242] Compound 46-63
[0243] Synthesis method: Dissolve 1 mmol of substituted phenylthiourea and 1 mmol of 2-chloroacetylacetone (ethyl 2-chloroacetoacetate) in 20 mL of methanol, reflux overnight, spin dry methanol, add a small amount of dilute potassium carbonate aqueous solution to neutralize, and saturate It was washed with brine, extracted with ethyl acetate, and the organic layer was concentrated and dried to obtain the product by column chromatography.
[0244] Target compound spectral data
[0245] 2-(4-Methyl-3-chloroanilino)-4-methyl-5-acetylthiazole
[0246]
[0247] 1 H NMR (400MHz, DMSO) δ (ppm): 7.79(d, J=2.0Hz, 1H), 7.48-7.38(m, 1H), 7.30(d, J=8.0Hz, 1H), 2.55(s, 3H ), 2.42(s, 3H), 2.27(s, 3H).
[0248] HRMS (ESI) calcd for C 13 h 13 ClN 2 OS(M+H + )281.0515, found 281.0515;
[0249] 2-(3,4-Methylanilino)-4-methyl-5-acetylthiazole
[0250]
[0251] 1 H NMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com