Synthetic method of 2-(N-alkyl)aminooimidazole derivatives
A technology of aminoimidazole and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of low atomic efficiency and environmental pollution, and achieve the effect of high economic efficiency and broad development prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of N-benzyl-1H-benzimidazol-2-amine
[0025] N-benzyl-1H-benzo[d]imidazol-2-amine
[0026]
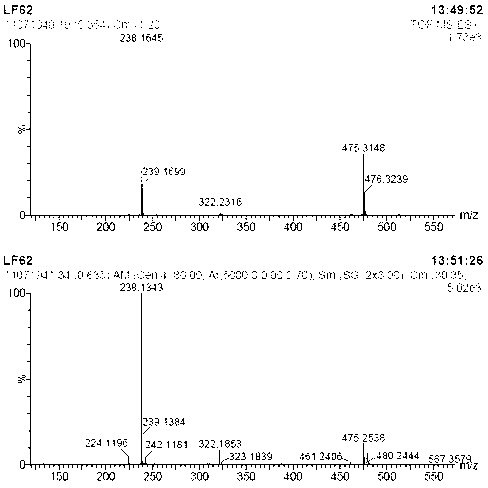
[0027] Under nitrogen protection, 2-aminobenzimidazole ( 133 mg, 1 mmol), [Cp*IrCl 2 ] 2 (1.6 mg, 0.002 mmol, 0.2 mol%), potassium carbonate (13.8 mg, 0.1 mmol, 10 mol%) and benzyl alcohol (432 mg, 4 mmol) were sequentially added to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 96%, and its product NMR spectrum and high-resolution mass spectrum were as follows: figure 1 and figure 2 as shown,
[0028] 1 H NMR (500 MHz, DMSO-d 6 ) δ 7.37 (d, J = 7.3 Hz, 2H, ArH), 7.32 (t, J = 7.6 Hz, 2H, ArH), 7.23 (t, J = 7.2 Hz, 1H, ArH), 7.13-7.11 (m, 2H, ArH), 6.86 (dd, J = 5.7 Hz and 3.2 Hz, 2H, Ar...
Embodiment 2
[0029] Example 2: Preparation of N-(4-methylphenyl)-1H-benzimidazol-2-amine
[0030] N-(4-methylbenzyl)-1H-benzo[d]imidazol-2-amine
[0031]
[0032] Under nitrogen protection, 2-aminobenzimidazole ( 133 mg, 1 mmol), [Cp*IrCl 2 ] 2(1.6 mg, 0.002 mmol, 0.2 mol%), potassium carbonate (13.8 mg, 0.1 mmol, 10 mol%) and 4-methylbenzyl alcohol (488 mg, 4 mmol) were sequentially added to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 84%, and its product NMR spectrum and high-resolution mass spectrum were as follows: image 3 with Figure 4 as shown,
[0033] 1 H NMR (500 MHz, DMSO-d 6 ) δ 10.77 (s, 1H, NH), 7.26 (d, J = 7.4 Hz, 2H, ArH), 7.12 (d, J = 7.1Hz, 4H, ArH), 6.87 (s, 1H, ArH), 6.83 (s, 1H, ArH), 4.45 (br s, 2H, CH 2 N...
Embodiment 3
[0034] Embodiment 3: Preparation of N-(4-methoxyphenyl)-1H-benzimidazol-2-amine
[0035] N-(4-methoxybenzyl)-1H-benzo[d]imidazol-2-amine
[0036]
[0037] Under nitrogen protection, 2-aminobenzimidazole ( 133 mg, 1 mmol), [Cp*IrCl 2 ] 2 (1.6 mg, 0.002 mmol, 0.2 mol%), potassium carbonate (13.8 mg, 0.1 mmol, 10 mol%) and 4-methoxybenzyl alcohol (552 mg, 4 mmol) were sequentially added to a 20 ml Schlenk reaction flask. The reaction mixture was at 130 o After 12 h at C, cool to room temperature. The solvent was removed by rotary evaporation, and then the pure target compound was obtained by column chromatography (developing solvent: ethyl acetate / petroleum ether), yield: 87%
[0038] 1 H NMR (500 MHz, DMSO-d 6 ) δ 10.75 (s, 1H, NH), 7.30 (d, J = 7.7 Hz, 2H, ArH), 7.11 (d, J = 6.1 Hz, 2H, ArH), 7.01 (br s, 1H, NH), 6.88 (m, 4H, ArH), 4.42 (s, 2H, CH 2 N), 3.71 (s, 3H, OCH 3 ); HRMS-EI (70 eV) m / z calcd for C 15 h 16 N 3 O [M+H] + 254.1293, found 254.1300...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com