Controlled release compositions with reduced food effect
A food effect and composition technology, applied in the direction of drug combination, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of not being clinically effective, poor drug absorption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] A preparation of controlled release composition
[0082] A controlled release tablet containing 1000 mg of metformin hydrochloride is prepared as follows:
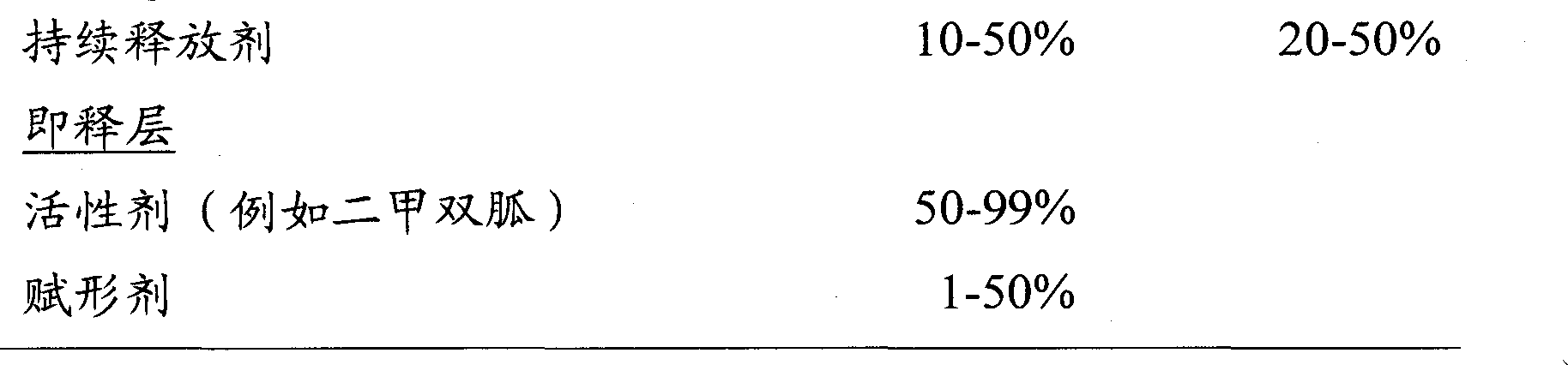
[0083] (a) Sustained release layer
[0084]
[0085] HPMC K100LV CR was dissolved in pure water as a binder solution. Metformin hydrochloride, Na 2 HPO 4 Mix with a portion of HPMC K100M CR (inside) and pass through a 30 mesh screen. The blended powders were wet granulated by sparing with the binder solution in a high shear mixer. The granules were dried at 70°C in a fluid bed granulator until the loss on drying did not exceed 3%. Pass the dried granules through a Comil fitted with a 20# mesh screen. HPMC K100M CR (external) was passed through a 30# mesh screen and mixed with the dry granules through a V-blender. Magnesium stearate was passed through a 30# mesh screen and blended with the mixture.
[0086] (b) Instant release layer
[0087]
[0088] Will EF was dissolved in pure water as a binder so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com