Isoquinoline drug, its preparation and application
A technology of isoquinolines and derivatives, applied in the field of medicine, can solve the problems of berbamine hydrochloride crystalline hydrate that have not been published in the literature, and achieve the effects of improving operability, facilitating storage and transportation, and preventing adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
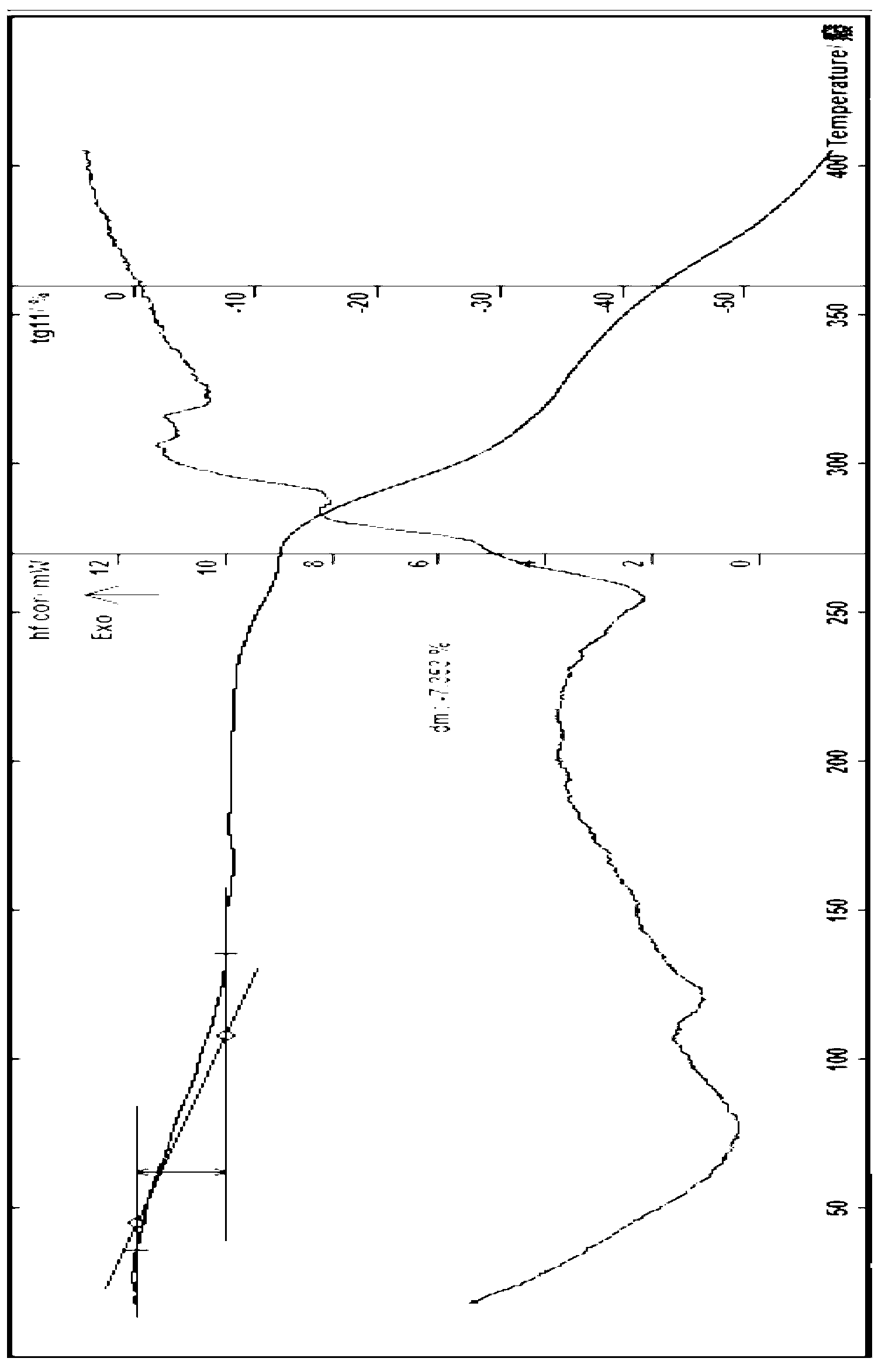
[0065] Embodiment 2 (attached Figure 4 ), at the positions of the following 2θ values of about 6.37, 7.30, 9.28, 10.32, 11.15, 11.67, 12.71, 13.36, 13.80, 14.40, 15.04, 15.64, 16.66, 17.16, 18.30, 19.26, 19.92, 20.71, 21.20, 243.62 , 25.46, 26.64, 27.14, 27.86, 29.04, 29.97, 30.53, 31.90, 33.45 etc. have characteristic peaks,
[0066] attached Figure 4 Where the above data is unclear, the following data can be seen, and the details are as follows:
[0067] PEAK: 21-pts / Parabolic Filter, Threshold=3.0, Cutoff=1.0%, BG=3 / 1.0, Peak-Top=Centroid Fit
[0068]
[0069]
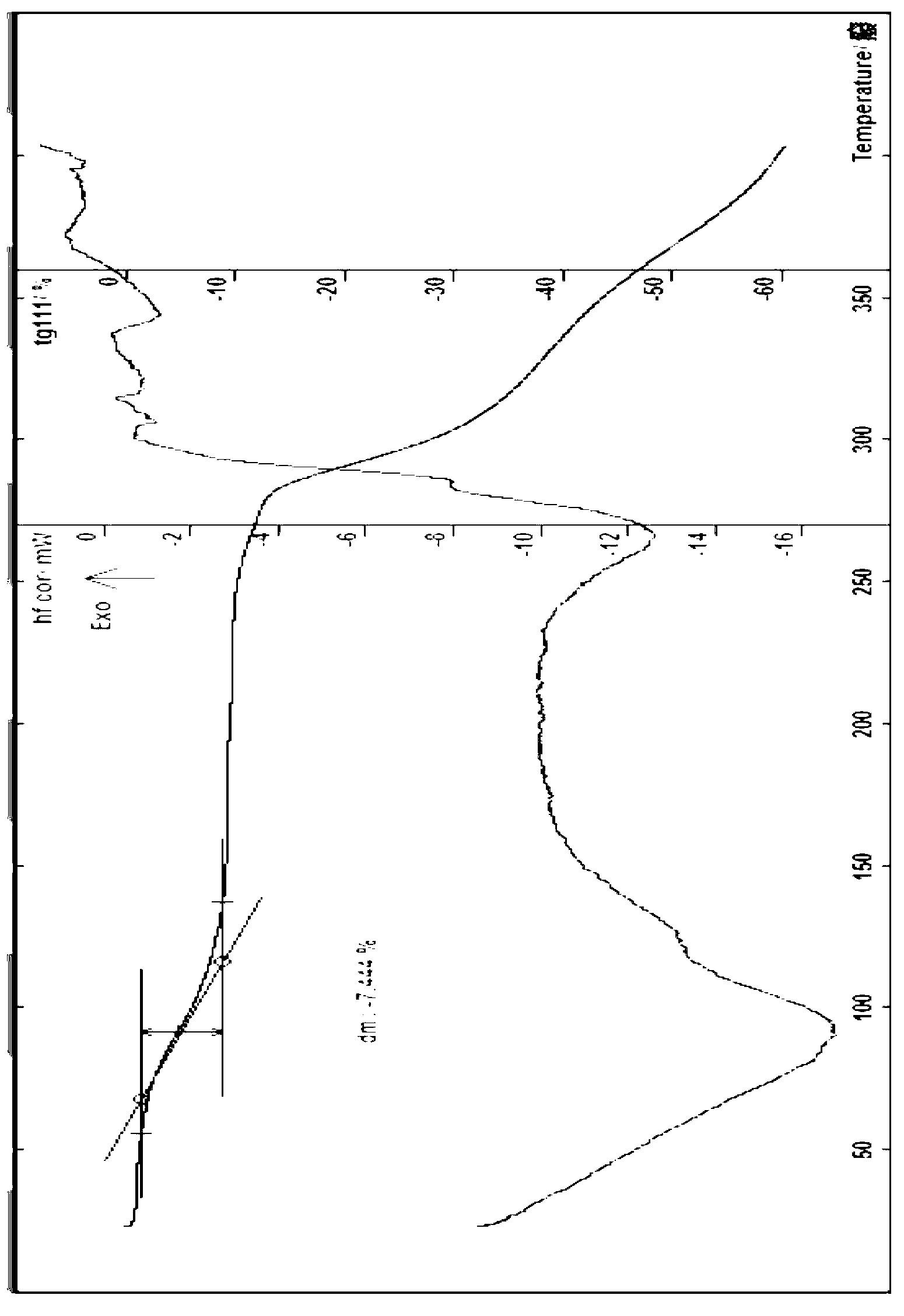
[0070] In another embodiment (Example 3), measured by powder X-ray diffraction method, within the measurement range of diffraction angle 2θ (3-60°), berbamine hydrochloride 3.5 hydrate of the present invention can include the following 2θ values The positions have corresponding eigenvalues, about 6.31, 6.84, 7.24, 11.05, 11.61, 12.67, 13.77, 14.31, 14.83, 15.59, 16.56, 17.13, 18.20, 19.02, 19.62, 20.69,...
Embodiment 1
[0071] Example 1 Preparation of berbamine hydrochloride trihydrate In a 250ml flask, add 60ml of water, 10g of berbamine hydrate, add 6M hydrochloric acid solution, stir to dissolve, control the pH of the solution to about 1.5-3.5, and continue stirring for 30 minutes Add an appropriate amount of activated carbon, stir, filter, concentrate the filtrate, add an appropriate amount of isopropanol, cool, leave to precipitate solid crystals, filter, and dilute the obtained solid at about 30°C for about 2 days, then place it in a container with a color-changing silica gel desiccant Dry in a glass desiccator at room temperature of about 20°C, and dry in vacuum at about 45°C (the reading of the vacuum gauge in the vacuum drying oven is about 0.08MPa) for 6 hours to obtain 8.3g of off-white to light yellow crystalline powder. Identification: (1) Take about 10 mg of the product of the present invention, add 5 ml of water to dissolve it, put it in two test tubes, add 1 drop of bismuth pot...
Embodiment 3
[0073] Example 3 Preparation of berbamine hydrochloride 3.5 hydrate Take 12.6g of berbamine, add 50ml of ethanol and 5ml of water in a 250ml flask, add hydrochloric acid solution with a concentration of about 6M, stir to make the solution pH=1.0-3.5 after dissolution , continue stirring for about 30 minutes, after concentration, add an appropriate amount of isopropanol, place it, cool it to about 0°C, place it, wait until the precipitate is fully separated, filter, and recrystallize the obtained solid twice with an appropriate amount of water, isopropanol, and acetone , cooled, placed, until the solid is fully separated, filtered, the obtained solid is diluted and dried at about 30°C for about 1 day, and then placed in a glass desiccator with a color-changing silica gel desiccant and dried at room temperature at about 20°C, the obtained solid The product was dried at about 42°C for 6 hours to obtain 6.1 grams of off-white solid; identification: (1) Take about 10 mg of the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com