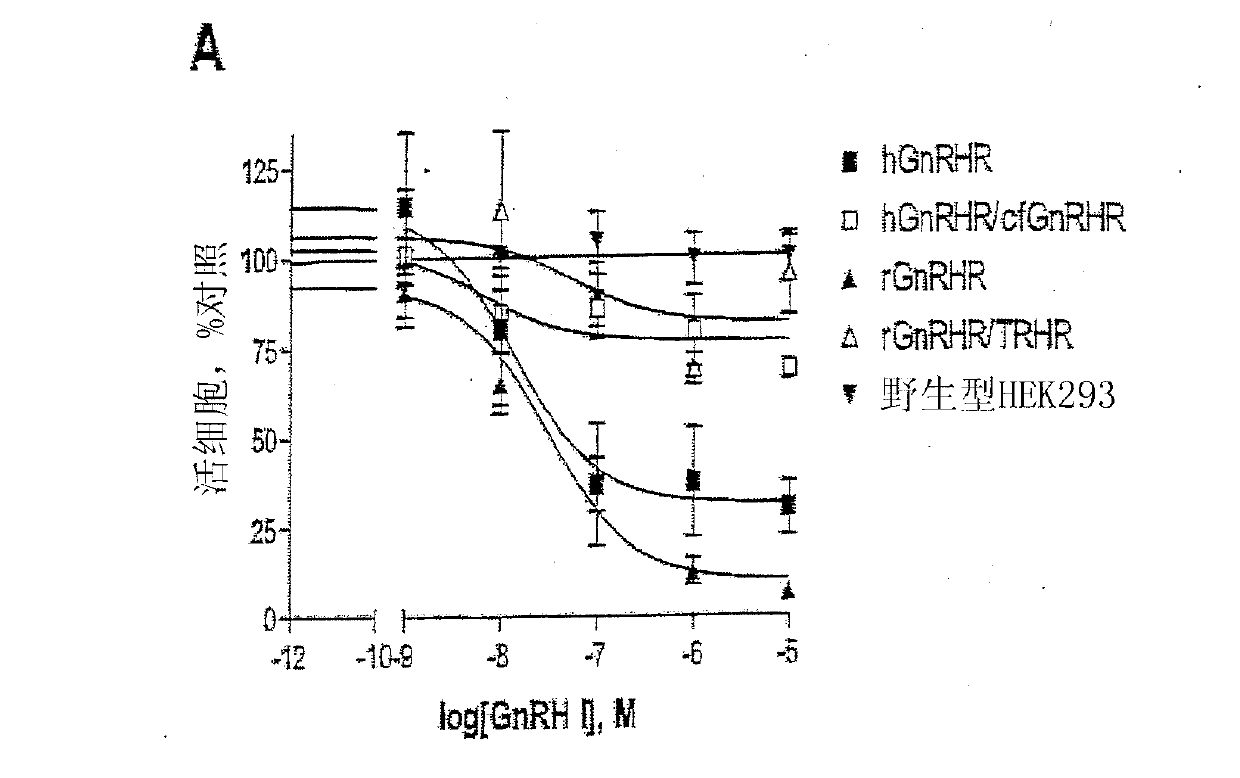
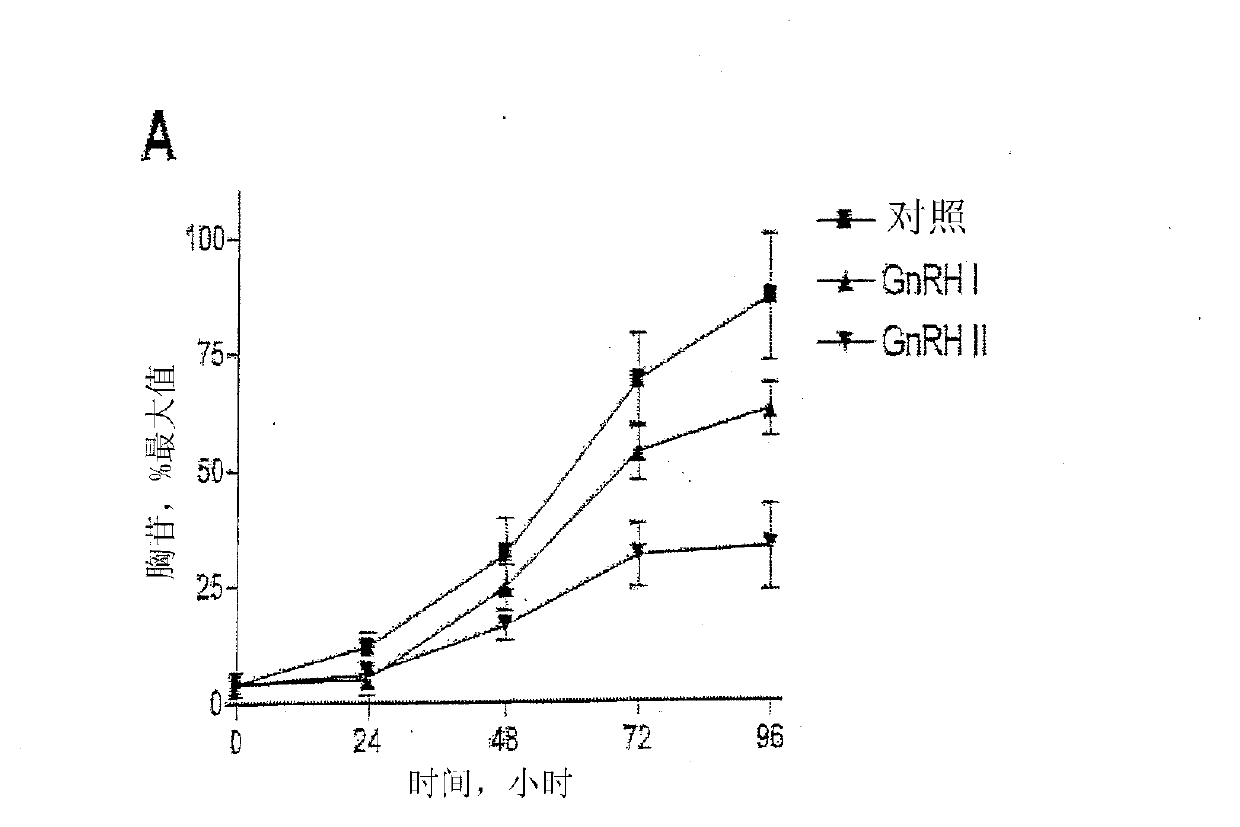
GNRH (gonadotropin-releasing hormone) peptide variants
A technology of drugs and peptide sequences, applied in the directions of luteinizing hormone-releasing hormone, biochemical equipment and methods, and microbial determination/inspection, etc., can solve the problem of not finding functional alternative subtypes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0254] Embodiment 1-experimental data
[0255] Materials and methods
[0256] Material
[0257] GnRH I and GnRH II were purchased from Sigma-Aldrich Co. Ltd. (Poole, Dorset, UK). Modified GnRH analogs were purchased from Roger Roeske (University of Indiana, Indianapolis, USA). Anti-phospho-ERK1 / 2 and anti-ERK2 antisera were purchased from New England Biolabs (UK) Ltd. (NEB; Hitchin, Herts, UK). Anti-cleaved poly[ADPribose] polymerase (PARP) antibody (Asp214 / Gly215; human specific) was purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA). All secondary antibodies were purchased from Sigma. Chemical inhibitors JNK inhibitor II, PD98059 (MEK inhibitor), SB203580 (p38MAPK inhibitor), wortmannin (PI-3K inhibitor), herbimycin A (tyrosine kinase Inhibitor), PP2 (Src Inhibitor), AG1478 (EGFR Inhibitor), U-73122 (PLC Inhibitor), Ro-31-8220 (PKC Inhibitor) and (PKC inhibitor) was purchased from Calbiochem (Calbiochem / Merck Biosciences Ltd.; Nottingham, East Midland...
Embodiment 2
[0308] Example 2 Exemplary Pharmaceutical Composition
[0309] While it is possible to use the agents of the invention alone, it is preferred to present them as pharmaceutical compositions together with one or more acceptable carriers. The carrier must be "acceptable" in the sense of being compatible with the agents of the invention and not deleterious to its recipients. Usually, the carrier is sterile and pyrogen-free water or saline.
[0310] The following examples illustrate medicaments and pharmaceutical compositions according to the present invention, wherein the active ingredient is an agent of the present invention.
[0311] Preferably, the agent of the invention is provided in an amount ranging from 10 μg to 500 mg. It should be recognized that the following exemplary medicaments and pharmaceutical compositions can be formulated with 10 [mu]g to 500 mg of the agents described herein. For example, the agents of the invention may be presented in one-tenth or one-hundr...
Embodiment 3
[0367] Example 3 - Treatment of Proliferative Disorders Using Agents of the Invention
[0368] According to the method of the present invention, 1 mg of the agent of the present invention is administered intramuscularly per day to a patient with prostate cancer unresponsive to antiandrogen therapy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com