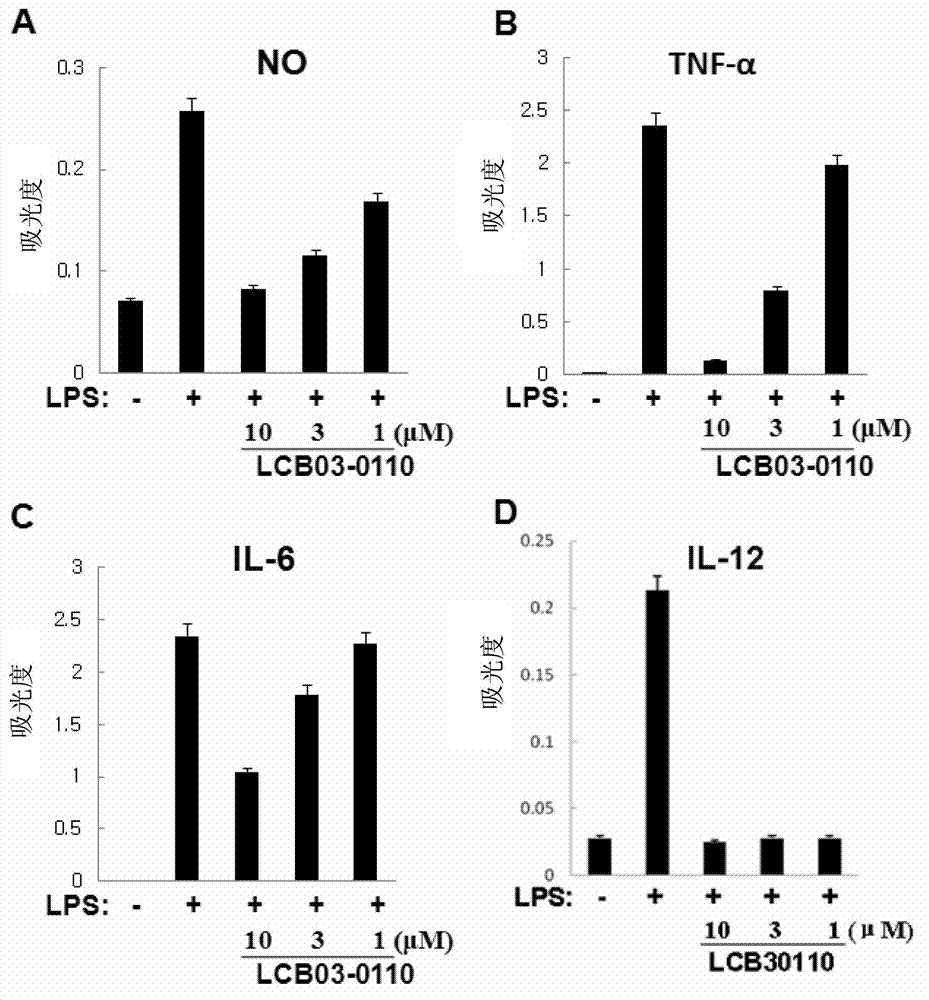
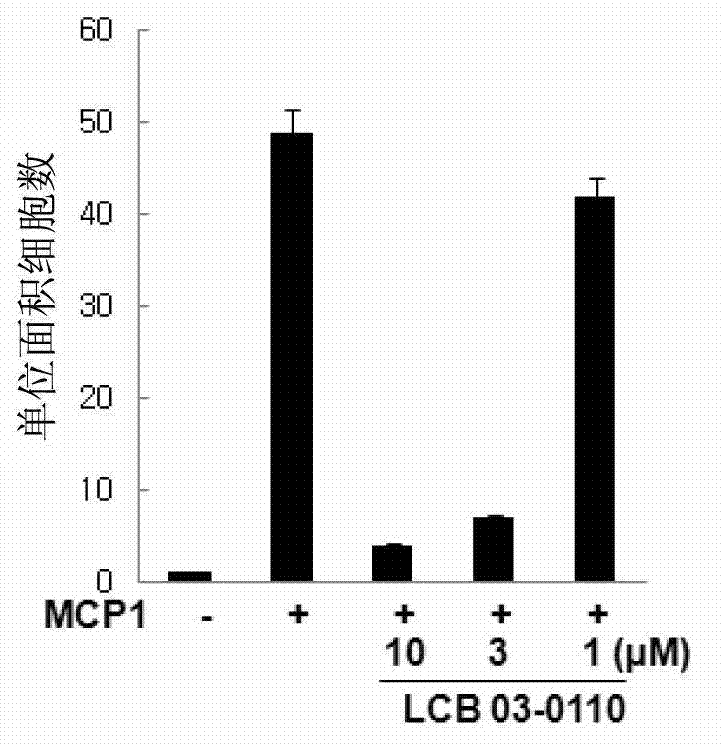
Anti-inflammatory compound having inhibitory activity against multiple tyrosine kinases, and pharmaceutical composition containing same
A compound and pharmaceutical technology, applied in the field of anti-inflammatory compounds with various tyrosine kinase inhibitory activities and pharmaceutical compositions containing these compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0214]
preparation example 1-1
[0216] Synthesis of 1-(4-(methoxy-benzyloxy)-3-nitrobenzene
[0217] Add sodium hydroxide (0.54 g, 13.36 mmol) to 10 ml of degassed dimethylformamide at 0 °C, and then slowly dropwise add 3-nitrophenol (1.69 g, 12.14 mmol) dissolved in 7 ml of di Methylformamide, then p-methoxybenzyl chloride (1.81ml, 13.36mmol) was added slowly. After raising the reaction temperature to room temperature and stirring for 2 hours, the reaction mixture was extracted with saturated ammonium chloride (100 ml) and ethyl acetate (100 ml). The organic layer was washed twice with 100 ml of water, dried over anhydrous sodium sulfate, and then concentrated under vacuum. Recrystallization from n-hexane finally gave 1-(4-methoxybenzyloxy)-3-nitrobenzene (2.85 g, 90%) as a pale yellow solid.
[0218] 1 H-NMR(600MHz, CDCl3);δ7.83-7.81(m,2H),7.42(t,J=8.4Hz,1H),7.37(d,J=8.4Hz,2H),7.28(dd,J, 2.4,8.4Hz,1H),6.93(d,J=8.4Hz,2H),5.06(s,2H),3.82(s,3H)
preparation example 1-2
[0220] Synthesis of 3-(4-methoxybenzyloxy)aniline
[0221] Iron (5.6 g, 100.28 mmol) was mixed with methanol:water (15 ml:3 ml) and refluxed for 30 minutes. When the temperature dropped to room temperature, 1-(4-methoxybenzyloxy)-3-nitrobenzene (1.3 g, 5.01 mmol) was dissolved in 6 mL of methanol:water (5:1) solution, and after adding Reflux for 15 hours. Fe was removed with cellite, then the reaction mixture was washed with methanol and concentrated under vacuum to remove solvent. After extraction with 100 ml of saturated ammonium chloride + 150 ml of dichloromethane, the organic layer was washed with water. The organic solvent was concentrated in vacuo to give the title compound (1.14 g, 99%).
[0222] 1 H-NMR (400MHz, CDCl3); 6.31(m,3H),4.94(s,2H),3.81(s,3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com