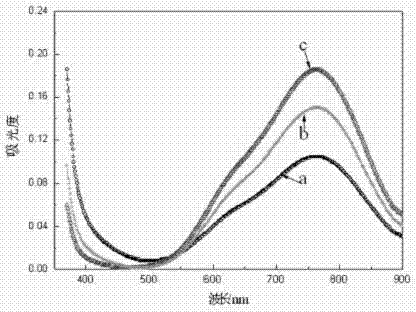
Method for preparing positive electrode electrolyte of vanadium battery
A cathode electrolyte and electrolyte technology, applied in the direction of regenerative fuel cells, etc., can solve the problems of difficult removal of impurities, unfavorable evaporation and crystallization, and difficult removal of oxalic acid, and achieve the effects of saving equipment investment, stable product quality, and reducing energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
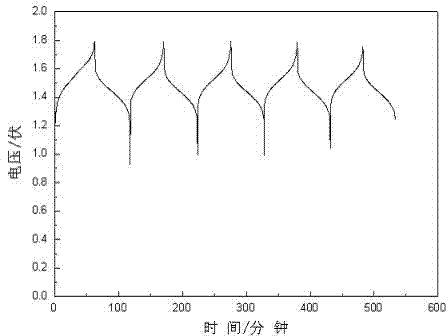
[0025] Take 1.2 g of C with a purity of 99%, V with a purity of 98% 2 o 5 18.2 g, 40 g of concentrated sulfuric acid with a specific gravity of 1.84g / mL, dilute the sulfuric acid with distilled water at a ratio of 1:1, add V 2 o 5 Then gradually add C while stirring, filter after cooling, evaporate the solution, add 2 mol / L sulfuric acid solution, and obtain a positive vanadium electrolyte that can be used for vanadium batteries. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7 V, and the discharging platform is 1.4 V.
Embodiment 2
[0027] Take 24 g of C with a purity of 99%, V with a purity of 98% 2 o 5 364 g, 800 g of concentrated sulfuric acid with a specific gravity of 1.84g / mL, dilute the sulfuric acid with distilled water at a ratio of 1:1, add V 2 o 5 Then gradually add C while stirring, filter after cooling, evaporate the solution, add 2 mol / L sulfuric acid solution, and obtain a positive vanadium electrolyte that can be used for vanadium batteries. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7 V, and the discharging platform is 1.4 V.
Embodiment 3
[0029] Take 240 g of C with a purity of 99%, V with a purity of 98% 2 o 5 3700 g, 8000 g of concentrated sulfuric acid with a specific gravity of 1.84g / mL, dilute the sulfuric acid with distilled water at a ratio of 1:1, add V 2 o 5 Then gradually add C while stirring, filter after cooling, evaporate the solution, add 2 mol / L sulfuric acid solution, and obtain a positive vanadium electrolyte that can be used for vanadium batteries. Put the prepared electrolyte into the electrolytic cell for charging and discharging experiments: the charging platform is 1.7 V, and the discharging platform is 1.4 V.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com