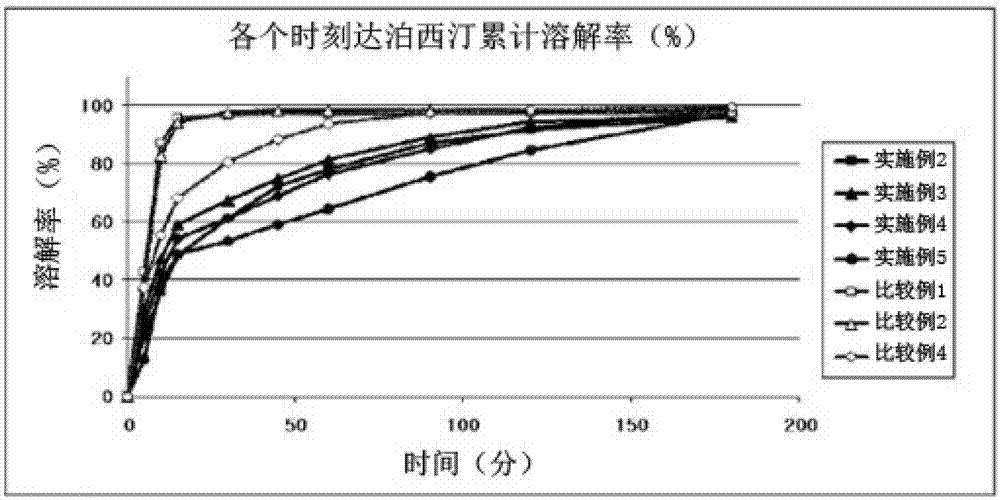
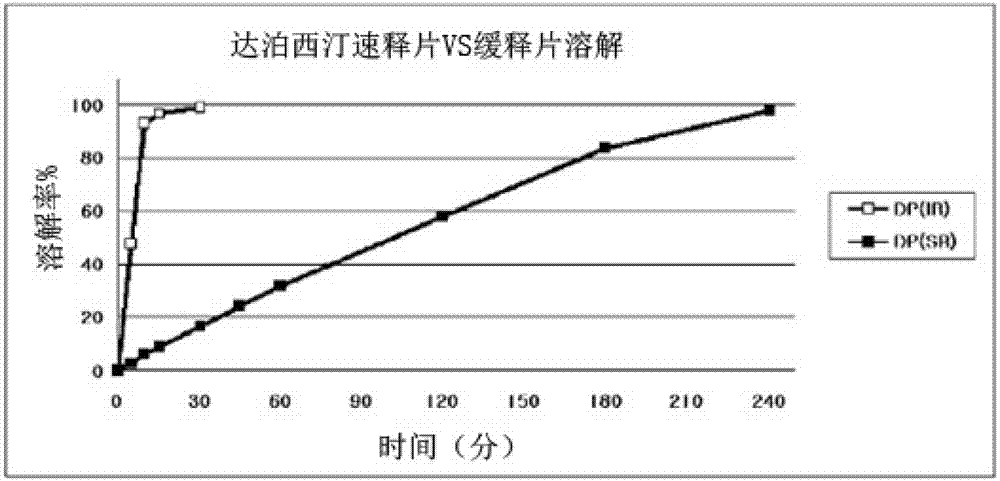
Time-delayed sustained release pharmaceutical composition comprising dapoxetine for oral administration
A technology of delayed release of dapoxetine, which is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc. It can solve the problems of inability to exert itself, different half-life in the blood, etc., and achieve the goal of satisfying sexual desire and reducing side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1, manufacturing a pharmaceutical composition (1): bilayer tablet form
[0046] 33.6 g of Dapoxetine HCI was mixed with 89.4 g of lactose hydrate (Supertab 14SD), 80.0 g of microcrystalline cellulose (Avicel Ph200) and 12.0 g of crospovidone (Kolidon CL) before adding 1.0 g Magnesium stearate slip blend creates an immediate release blend. In addition, after mixing 33.6 g of dapoxetine hydrochloride, 24.4 g of lactose hydrate (Supertab 14SD), 75.0 g of hydroxypropyl methylcellulose (Methocel E50), and 35 g of polyvinylpyrrolidone VA64 (Kolidon VA64), 1.0 g was added Magnesium stearate creates an extended release blend. Each layer of each tablet contained 216 mg of the immediate-release mixture and 169 mg of the sustained-release mixture, and each layer contained 30 mg of each tablet, and a total of 60 mg of dapoxetine was manufactured. Then, it was coated with a coating solution prepared by dissolving the white film coating in purified water so that each tabl...
Embodiment 2
[0047] Example 2, Manufacture of Pharmaceutical Composition (2): Dosage Form of Mixed Tablets
[0048] 100.8g dapoxetine hydrochloride was mixed with 148.2g lactose hydrate (Pharmatose200), 300.0g microcrystalline cellulose (Avicel Ph101) and 36.0g croscarmellose sodium (Ac-Di-Sol), and then used The mixed solution prepared by dissolving 12.0 g of povidone (Kolidon K-30) in purified water was mixed to form particles and then dried. Then, 3.0 g of magnesium stearate was added after ingot into granules with a tablet machine to produce immediate-release granules. In addition, after mixing 100.8 g of dapoxetine hydrochloride with 316.2 g of lactose hydrate (Pharmatose 200) and 135.0 g of hydroxypropylmethylcellulose (Methocel E50), it was mixed with 15.0 g of povidone (Kolidon K-30) in The combined solution made by dissolving pure water is mixed to make granules and dried. Then 3.0 g of magnesium stearate was added by grading and mixed to manufacture sustained-release granules...
Embodiment 3
[0049] Embodiment 3, manufacture pharmaceutical composition (3): dosage form of bilayer tablet
[0050] The immediate-release mixture produced in Example 1 and the sustained-release granules produced in Example 2 were compressed with a double-layer tablet press so that each layer contained 216 mg and 169 mg, thereby producing each tablet with each layer Contains 30 mg bilayer tablet containing a total of 60 mg dapoxetine. Then, it was coated with a coating solution prepared by dissolving the white film coating in purified water so that each tablet contained 15 mg of the white film coating.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com