Diisocyanate having fluorine-containing branched chain, preparation method and application thereof
A diisocyanate and side chain technology, applied in the application field of water-based fluorine-containing polyurethane, can solve the problems of slow reaction, poor reactivity, and limitation of practical application, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A side chain fluorine-containing diisocyanate, calculated in parts by weight, its raw material composition and content are as follows:
[0043] 6 parts trimethylolpropane
[0044] Isophorone diisocyanate 100 parts
[0045] 2,2,2-Trifluoroethanol 5 parts
[0046] Catalyst 0.06 parts
[0047] Wherein said catalyst is stannous octoate.
[0048] The preparation method of the above-mentioned side chain fluorine-containing diisocyanate, the steps are as follows:
[0049] Stir and dehydrate 6 parts of trimethylolpropane at 110°C and a vacuum of 750mmHg for 0.5h, add 100 parts of isophorone diisocyanate and 0.03 parts of stannous octoate under nitrogen protection, react at 80°C for 2h, add 5 parts of 2 , 2,2-Trifluoroethanol and the remaining stannous octoate, continue to react at 80°C for 3h to obtain a light yellow viscous side chain fluorine-containing diisocyanate.
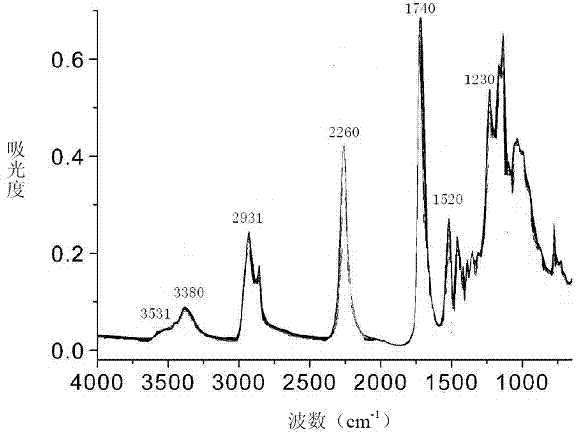
[0050] The side chain fluorine-containing diisocyanate of above-mentioned gained carries out infrared s...
Embodiment 2
[0077] A side chain fluorine-containing diisocyanate, calculated in parts by weight, its raw material composition and content are as follows:
[0078] Trimethylolpropane 10 parts
[0079] Isophorone diisocyanate 120 parts
[0080] 2,2,2-Trifluoroethanol 10 parts
[0081] Catalyst 1.8 parts
[0082] The catalyst described therein is a mixture of dibutyltin dilaurate and triethylamine in a mass ratio of 1:1.
[0083] The preparation method of the above-mentioned side chain fluorine-containing diisocyanate, the steps are as follows:
[0084] Stir and dehydrate 10 parts of trimethylolpropane at 110°C and a vacuum of 720mmHg for 1 hour, add 120 parts of isophorone diisocyanate and 0.9 parts of catalyst under nitrogen protection, react at 85°C for 2.5 hours, add 10 parts of 2,2 , 2-trifluoroethanol and the remaining catalyst, continue to react at 85°C for 2h, and obtain a water-white viscous side chain fluorine-containing diisocyanate.
[0085] A kind of aqueous fluorine-contai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com