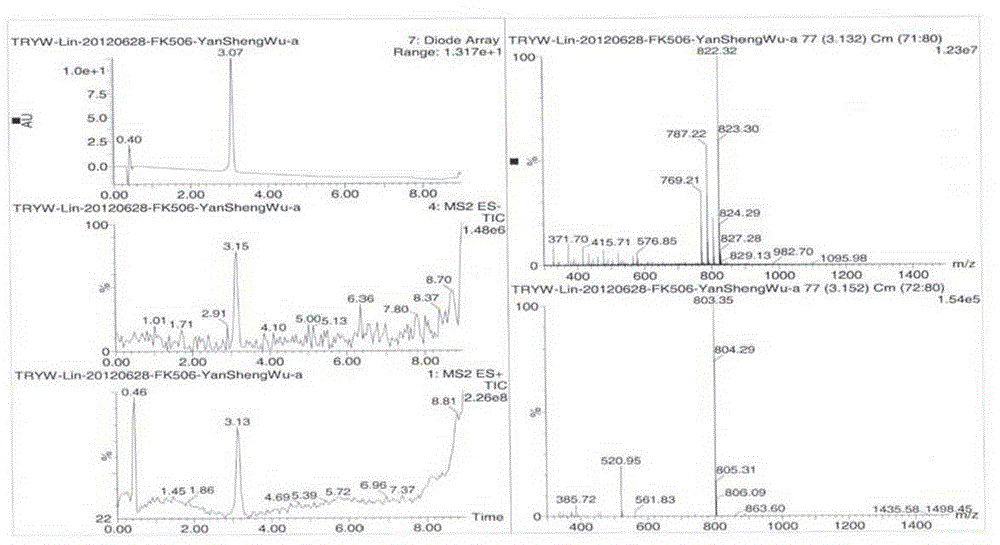
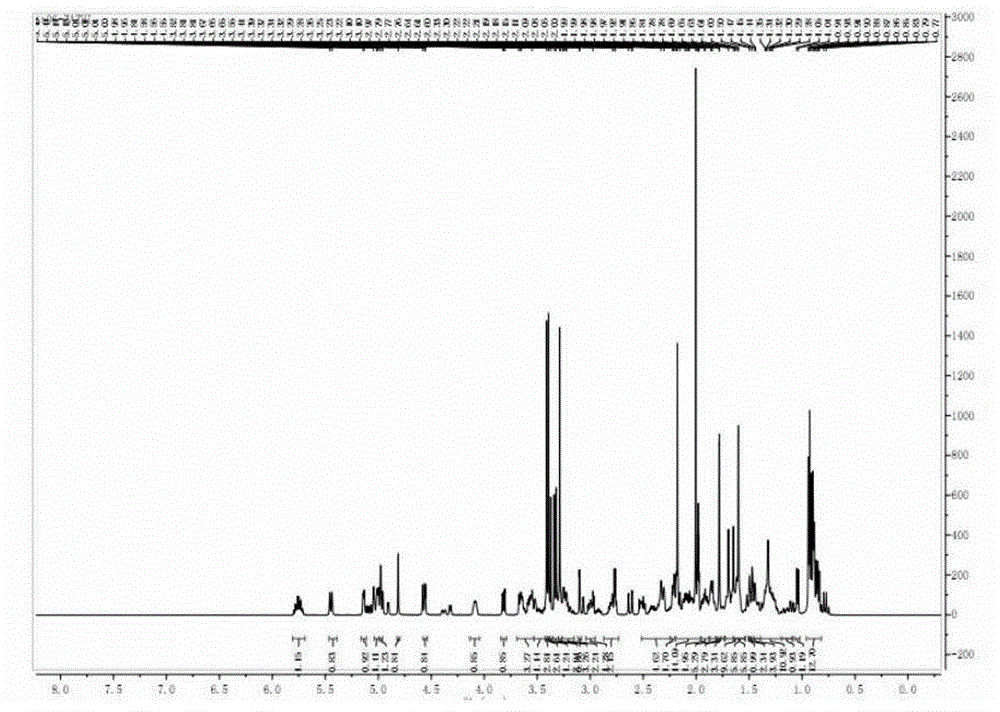
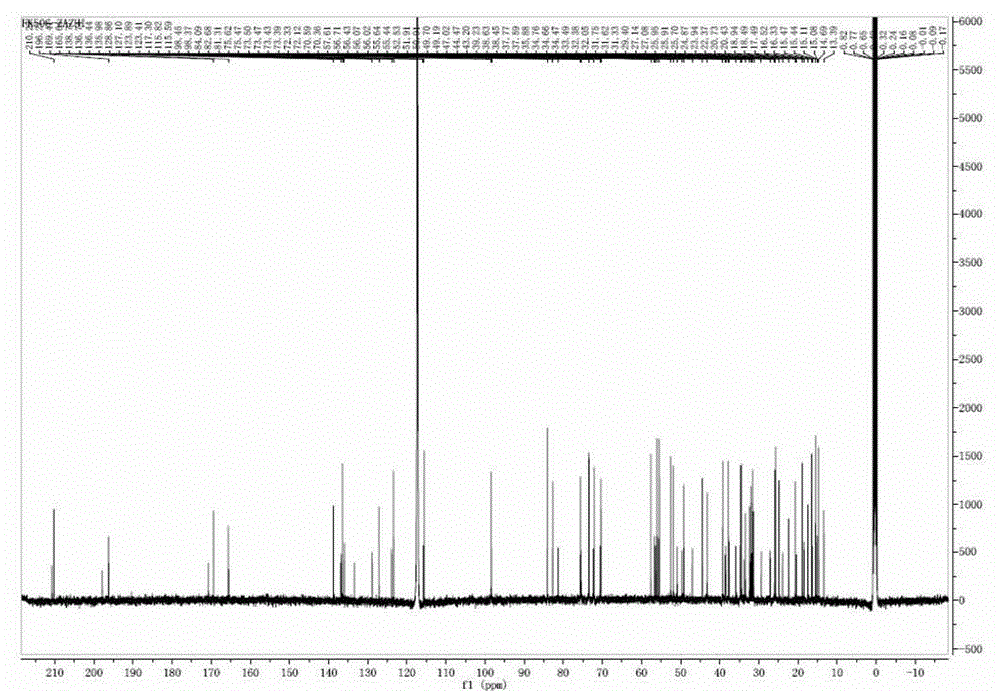
Preparing method of compound FK506-A
A technology of FK506-A, 1. FK506-A is applied in the field of preparation of macrolide compound FK506-A compound, can solve the problems of unstable products of FK506FK506 and the like, achieves simple operation, improved reaction efficiency and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a. Put 10g of FK506 into an evaporating dish and heat at 120°C for 15 minutes to obtain the crude product of FK506-A;
[0035] b. Add ethyl acetate to the FK506-A crude product, add 19g of 300-400 mesh silica gel after dissolving, stir evenly and then dry to obtain a dry sample;
[0036] c. Add the dry sample to a chromatographic column equipped with 80g of 300-400 mesh silica gel, add ether for elution, use HPLC to detect the concentration of FK506-A in the eluate, and collect and determine the purity of FK506-A ≥ 75 % of the eluate, concentrated to dryness at 30°C under reduced pressure to obtain 1.6g of solid, to each gram of solid, add 15ml of crystallization solvent consisting of acetone and water with a volume ratio of 4:1, heat to dissolve, then slowly cool down to crystallize After the crystals were no longer precipitated, suction filtered, and the filter cake was washed twice with the above crystallization solvent, and the filter cake was vacuum-dried at 60 ° C...
Embodiment 2
[0041] a. Put 10g of FK506 into an evaporating dish and heat at 130°C for 5 minutes to obtain the crude product of FK506-A;
[0042] b. Add ethanol to the FK506-A crude product, add 25g of 160-230 mesh silica gel after dissolving, stir evenly and then dry to obtain a dry sample;
[0043] c. Add the dry sample to a chromatographic column equipped with 150g of 160-230 mesh silica gel, add a mixture of isopropyl ether and acetone with a volume ratio of 8:1 to elute, and collect and test the purity of FK506-A ≥ 75 by HPLC. % of the eluate was concentrated to dryness at 60°C under reduced pressure to obtain 2.1 g of solid, and 5 ml of ether was added to each gram of solid. After heating and dissolving, the temperature was slowly cooled to crystallize. After the crystals were no longer precipitated, filter and wash with ether After the cake was caked once, the filter cake was vacuum-dried at 30°C to obtain 1.9 g of pure FK506-A, and the purity of FK506-A in the obtained FK506-A pure...
Embodiment 3
[0045] a. Put 10g of FK506 into an evaporating dish and heat at 110°C for 25 minutes to obtain the crude product of FK506-A;
[0046] b. Add acetone to the FK506-A crude product, add 10g of 100-200 mesh silica gel after dissolving, stir evenly and dry to obtain a dry sample;
[0047]c. Add the dry sample to a chromatographic column equipped with 120g of 100-200 mesh silica gel, add a mixture of n-hexane and acetone with a volume ratio of 10:1 to elute, collect and test the purity of FK506-A ≥ 75% by HPLC The eluate was concentrated to dryness at 50°C under reduced pressure to obtain 1.8 g of solids. To each gram of solids, 13 milliliters of crystallization solvents consisting of n-hexane and acetone with a volume ratio of 10:1 were added. After dissolving, the temperature was slowly cooled to crystallize. After the crystals are no longer precipitated, filter, wash the filter cake once with the above crystallization solvent, and dry the filter cake under vacuum at 40°C to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com