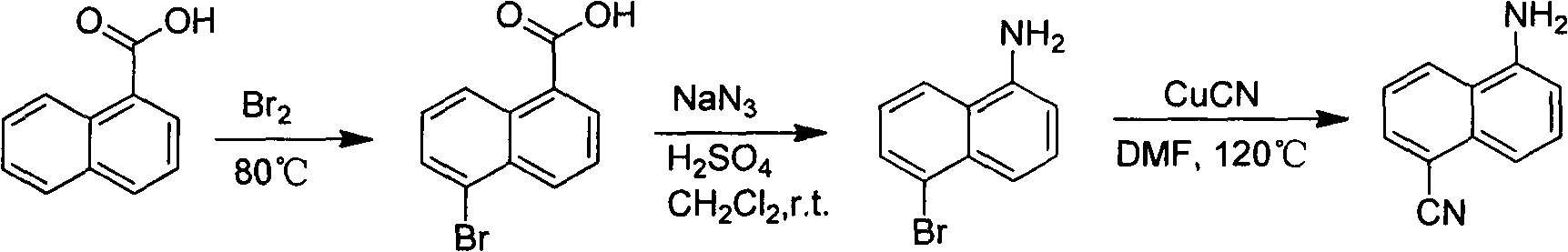Method for large-scale preparation of 5-amino-1-naphthyl nitrile
A technology of naphthalene nitrile and amino group, applied in the field of large-scale preparation of 5-amino-1-naphthalene nitrile, can solve the problems of harsh reaction conditions, high preparation cost, low yield, etc., and achieves low price, simple operation, safety, and reaction. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
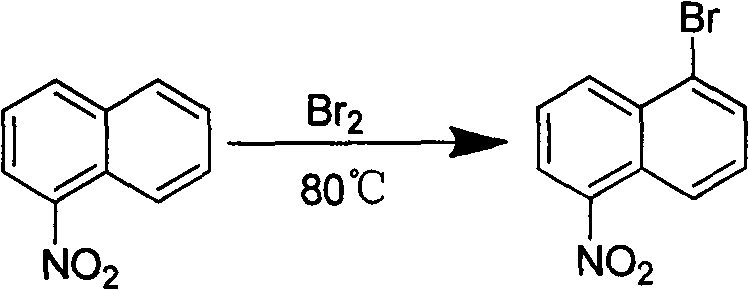
[0021] (1) Preparation of 1-bromo-5-nitronaphthalene:
[0022]
[0023] Heat 500g of nitronaphthalene (2.89mol) to 80-85°C to melt into a deep red liquid, keep the temperature constant and slowly add 450g of liquid bromine (2.81mol) dropwise within 2-3 hours. A large amount of acid gas released by the reaction is collected with lye. After one hour of reaction, the heating was stopped, and the reaction solution was poured into about 3 liters of ice water, and a large amount of yellow precipitate was precipitated, which was vacuum filtered to obtain a yellow solid, which was recrystallized with ethanol. 343 g of yellow needle crystals were obtained. Yield: 47%.
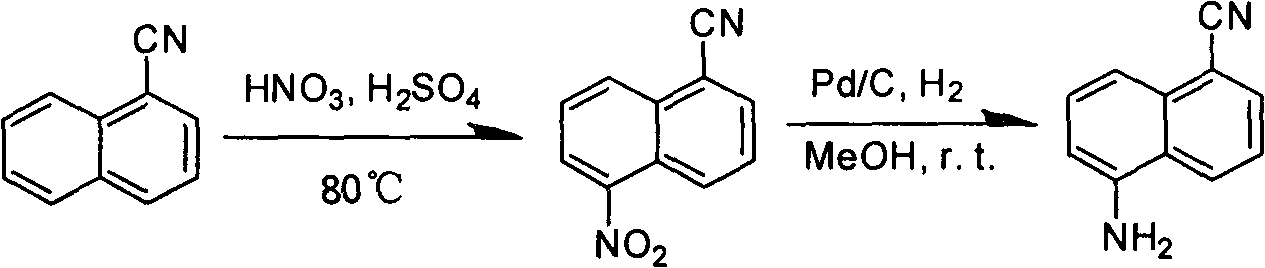
[0024] (2) Preparation of 5-nitro-1-naphthalene nitrile:
[0025]
[0026] Dissolve 343g (1.36mol) of 1-bromo-5-nitronaphthalene, 125g (1.4mol) of cuprous cyanide, and 193g (1.4mol) of potassium carbonate in 1500ml of N,N-dimethylformamide (DMF) , heated for 12 hours, the reaction solution was poured into a la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com