Hydrogenation method for activation of hydroxy-terminated butyronitrile catalyst
A liquid nitrile and terminal hydroxyl technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Liquid nitrile application fields, adding process steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
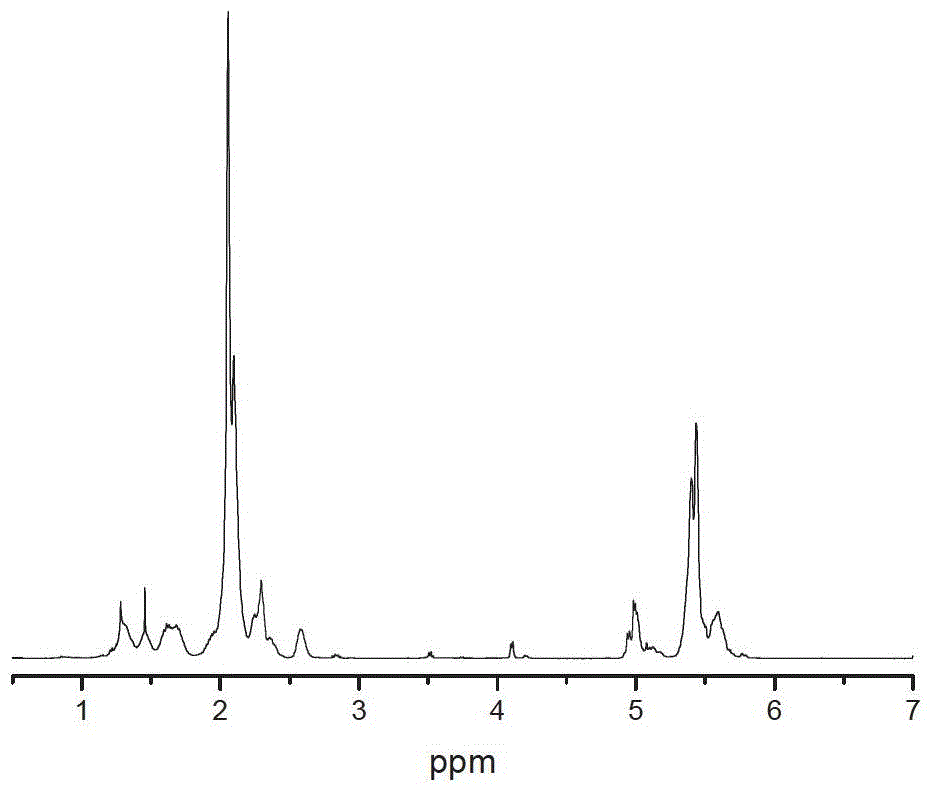
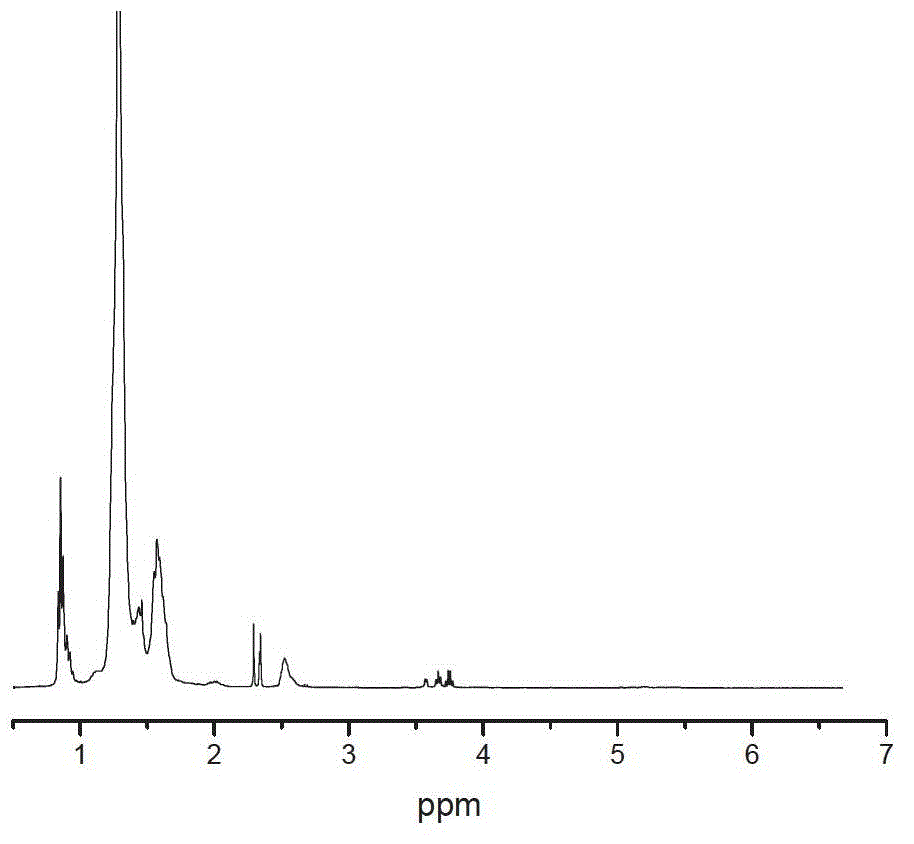
[0026] According to the above-mentioned general hydrogenation procedure, using hexamethyldiazide-capped liquid hydroxyl-terminated liquid butyronitrile (referred to as P-HTBN) as raw material, measure 150ML of hydroxyl-terminated liquid butyronitrile-butyronitrile xylene solution with a concentration of 16.7%, Put into a 0.5L reactor, catalyst rhodium chloride trihydrate 0.026g, cocatalyst methanol 10ml, control reaction temperature to 95°C, hydrogen pressure 3MPa, react for 6 hours, the results are shown in Table 1.
Embodiment 1-2
[0028] The raw material in Example 1-1 was changed to hydroxyl-terminated liquid butyronitrile (HTBN for short) without any capping and protection treatment, and other conditions were the same as in Example 1-1. The results are shown in Table 1.
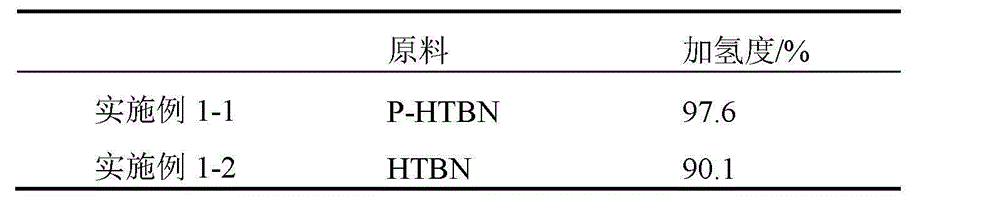
[0029] Table 1 Comparison of hydrogenation degree between P-HTBN and HTBN (with co-catalyst)
[0030]
[0031] The above examples show that the hydrogenation activity of P-HTBN is not much different from that of HTBN when the co-catalyst is added, and the addition of the co-catalyst has a significant activation effect on the catalyst.
Embodiment 2
[0041] Change the methanol in Example 1-1 to ethanol, isopropanol and acetone, and other results are the same as in Example 1-1, and the results are shown in Table 3.
[0042] Table 3 Effect of cocatalyst type on degree of hydrogenation
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com