Carrier suitable for cell transformation, recombinant cell and application thereof
A technology for transforming cells and recombining cells, applied in recombinant DNA technology, using vectors to introduce foreign genetic material, cells modified by introducing foreign genetic material, etc., can solve the problem of insensitivity to dioxin substances, high false positives, and British compounds need to be improved and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1 Construction of recombinant cell lines Hepa1-6DEGFP and Hepa1-6DLUC containing dioxin response element DRE and reporter gene
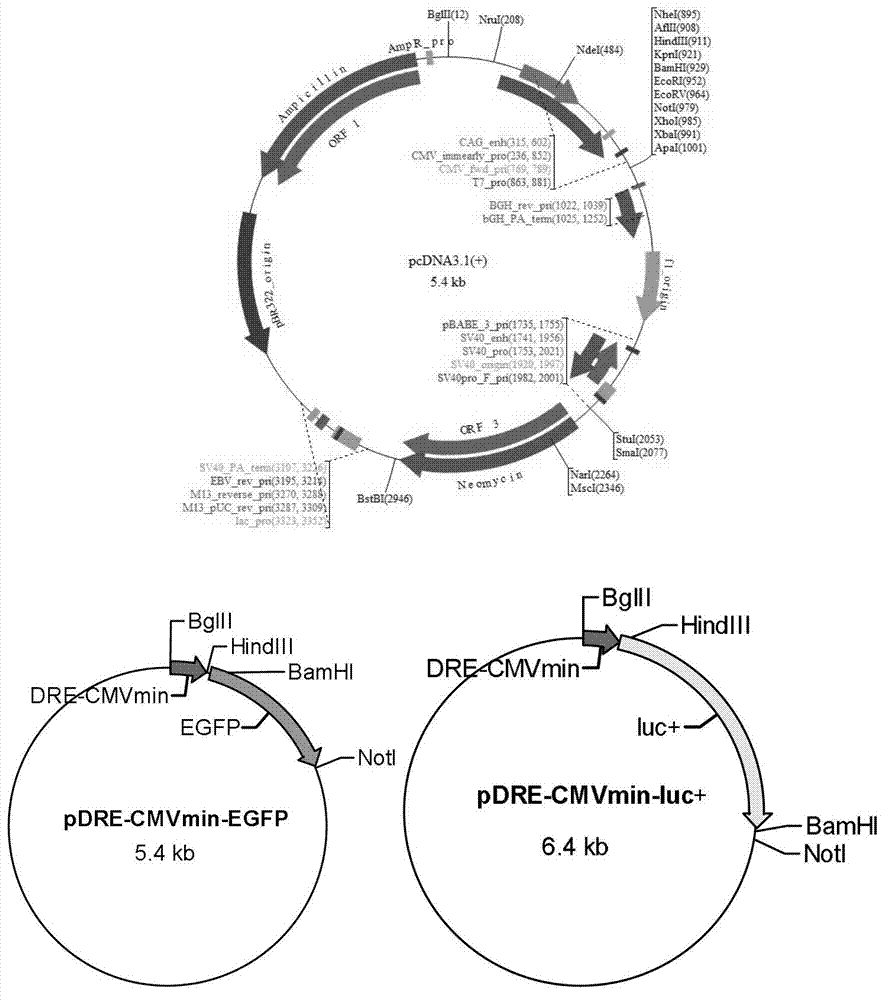
[0087] 1. Construction of recombinant vectors pDRE-CMVmin-EGFP and pDRE-CMVmin-luc+ containing dioxin response element DRE and reporter gene
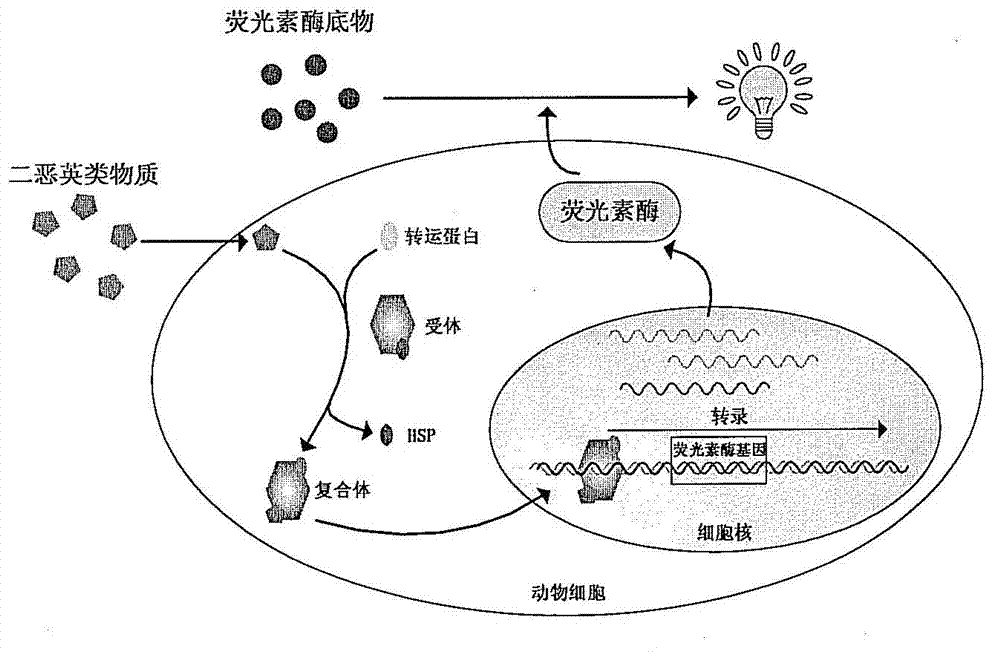
[0088] Studies have shown that the upstream of mouse cytochrome P450 gene CYP1A1 has six dioxin response elements labeled A-F, which can bind to the dioxin-aromatic hydrocarbon receptor complex to initiate the expression of CYP1A1, of which B, D, E, F High binding and activation efficiency (Amy Lusska et al. Protein-DNA Interactions at a Dioxin-responsive Enhancer. 1993. The Journal of Biological Chemistry.). Synthesize three tandem DRE sequences and the basic element CMVmin of the early promoter of cytomegalovirus (referred to as 3DRE+CMVmin in this article for convenience of description) by gene synthesis method (Invitrogen Company). Add BglII and HindIII restriction endonuclease sites to the...
Embodiment 2
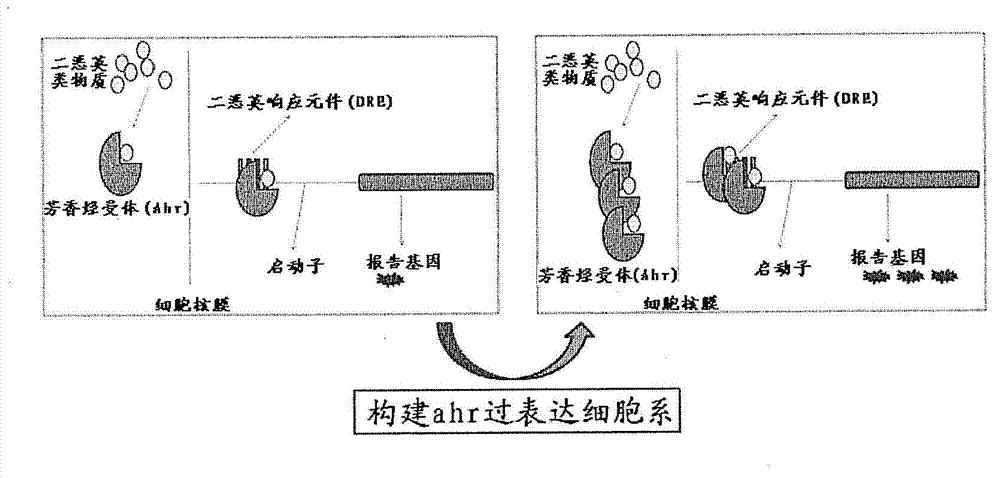
[0104] Example 2 Construction of high-sensitivity recombinant cell lines Hepa1-6ADEGFP and Hepa1-6ADLUC with ahr gene overexpression
[0105] 1. Aryl hydrocarbon receptor gene ahr overexpression vector construction
[0106] The coding sequence of the mouse aryl hydrocarbon receptor gene ahr was searched through NCBI, and its sequence number is NM_013464.4, which has the nucleotide sequence shown in SEQ ID NO:9. Primers were designed according to the sequence, and the primer sequences are shown in the table below:
[0107] Primer name
Primer sequence (5'-3', SEQ ID NO: )
P1
TAG ACCGGT ATGAGCAGCGGCGCCAACATC(10)
P2
CCG CTCGAG TCAACTCTGCACCTTGCTT(11)
[0108] Among them, P1 and P2 are used to amplify the ahr gene fragment. For the convenience of subsequent operations, P1 and P2 respectively contain AgeI and XhoI restriction endonuclease sites, which are underlined in the table.
[0109] The mouse liver was taken, and the total ...
Embodiment 3
[0139] Example 3 Comparison of Responses of Recombinant Cell Lines Hepa1-6DEGFP and Hepa1-6ADEGFP to Dioxin Standards (2,3,7,8-TCDD)
[0140] Inoculate the recombinant cell line in a 48-well cell plate, add 200 microliters of DMEM culture solution containing 10% FBS to each well, and place the cell plate at 37°C, 5% CO 2 Cultivate in a constant temperature incubator for 24 hours;
[0141] Dilute the dioxin standard 2,3,7,8-TCDD with DMSO, so that the final concentration of each well of the cell plate is 0.1pg / ml, each experimental group is repeated three times, and the control group uses the same volume of DMSO-treated cells;
[0142] Dioxin standards were mixed with recombinant cells and incubated for 24 hours. The green fluorescence was observed under a fluorescent microscope and photographed. The results are shown in Figure 5 middle. Such as Figure 5 As shown, two recombinant cells of Hepa1-6DEGFP and Hepa1-6ADEGFP emitted green fluorescence after being induced by dio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com