Novel protein and gene that codes therefor
A protein and coding technology, applied in the direction of anti-enzyme immunoglobulin, glycosylase, glycosyltransferase, etc., can solve the problems of no enzyme activity, no enzyme activity, loss of enzyme activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
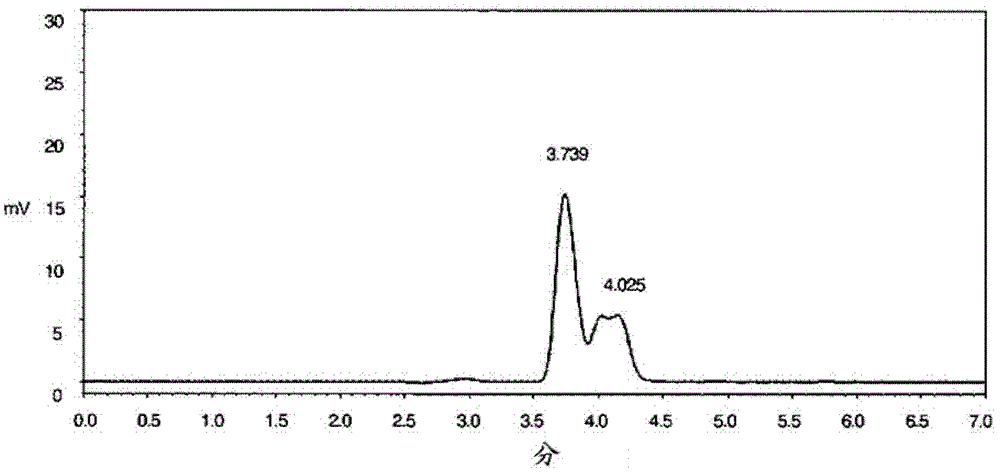
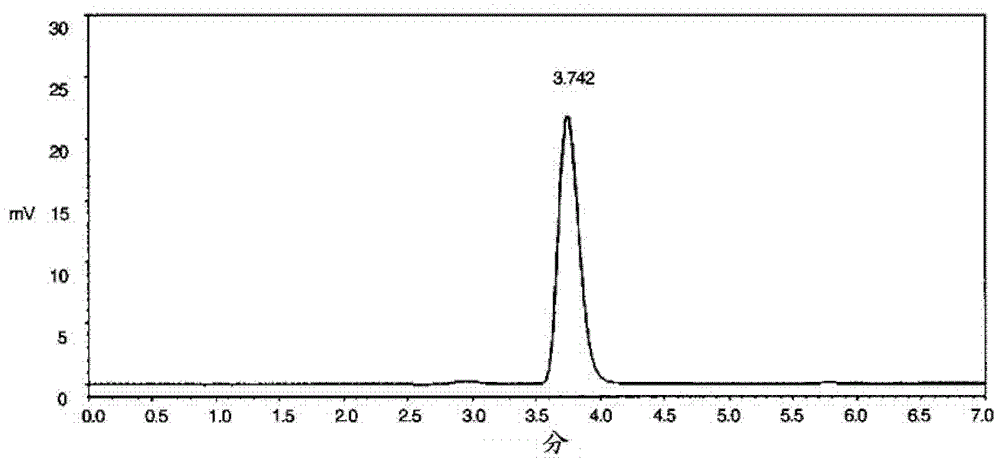
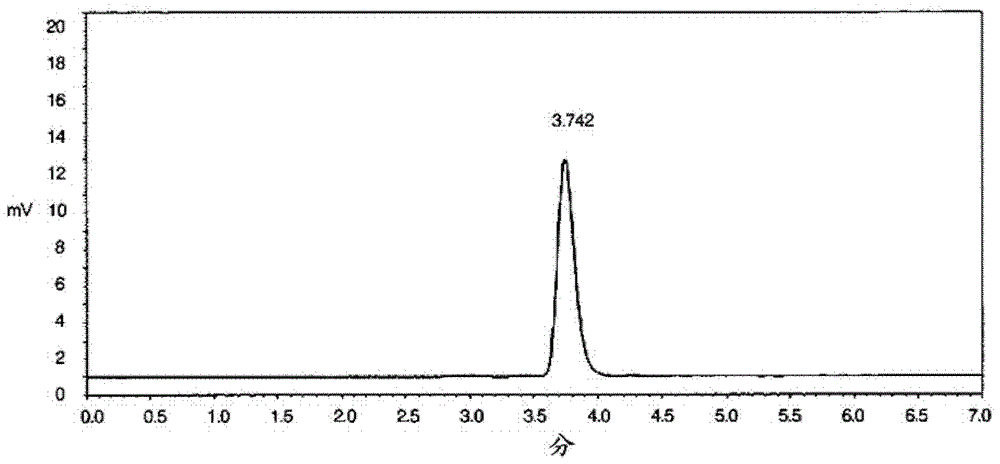
Image
Examples
Embodiment 1
[0154] Example 1: Screening and strains of microorganisms producing β-galactoside-α2,6-sialyltransferase Identification
[0155] (1) screening
[0156] Use seawater, sea sand, sea mud or marine fish and shellfish as the inoculation source. This inoculum source was spread on a plate medium composed of Marine Broth Agar 2216 medium (manufactured by Becton Dickinson), and microorganisms grown at 15°C, 25°C or 30°C were obtained. After the obtained microorganisms were purely cultured according to a conventional method, each microorganism was cultured using a liquid medium composed of Marine Broth 2216 medium (manufactured by Becton Dickinson). After the microorganisms grow sufficiently, the cells are collected from the culture medium by centrifugation. To the collected bacterial cells, 20 mM cacodylate buffer (pH 6.0) containing 0.2% Triton X-100 (manufactured by Kanto Chemical Industry Co., Ltd.) was added to suspend the bacterial cells. The cell suspension was ultrasoni...
Embodiment 2
[0162] Embodiment 2: Gram of β-galactoside-α2,6-sialyltransferase gene derived from JT-SHIZ-119 strain Long, base sequence determination, and the expression of the gene in Escherichia coli
[0163] (1) Confirmation of the homologue of the β-galactoside-α2,6-sialyltransferase gene in the JT-SHIZ-119 strain exist
[0164] In order to confirm the presence of β-galactoside-α2,6-sialyltransferase derived from Photobacterium mermaidi JT0160 strain in the JT-SHIZ-119 strain which has been confirmed to have β-galactoside-α2,6-sialyltransferase activity Enzyme gene (Yamamoto et al. (1996) J Biochem 120:104-110), or JT-ISH-224 strain derived β-galactoside-α2,6-sialyltransferase gene (PCT / JP2006 / 315850) For homologues, genomic Southern hybridization was performed.
[0165] First, in order to improve the efficiency of hybridization, an attempt was made to use the β-galactoside-α2,6-sialyltransferase gene fragment of the JT-SHIZ-119 strain itself as a probe. Specifically, it is u...
Embodiment 3
[0204] Example 3: From the large expression vector pTrc99A carrying the SHIZ119-N1C0 clone Extraction and purification of β-galactoside-α2,6-sialyltransferase from Enterobacter TB1
[0205] (1) Extraction and Purification
[0206] From the colony of Escherichia coli TB1 carrying the expression vector pTrc99A integrated with SHIZ119-N1C0 clone subcultured on the LBAmp plate medium, the bacterial cells were collected with an inoculation loop, and inoculated in a medium containing 30 μL × 200 ampicillin (400 mg / 20 mL) In 6 mL of LB liquid medium and 10 mL, shake culture at 30°C and 180 rpm for 8 hours.
[0207] The main culture was carried out according to the following procedure. Inject 300ml of LB medium mixed with 1.5mL x 200 ampicillin (400mg / 20mL) and 300μL of 1M IPTG (1.192g / 5mL) into a 1000ml baffled flask, and prepare 9 bottles in total (2.7L in total) . Each flask was inoculated with 12 ml of the pre-culture solution, and cultured with shaking at 30° C. and 180 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com