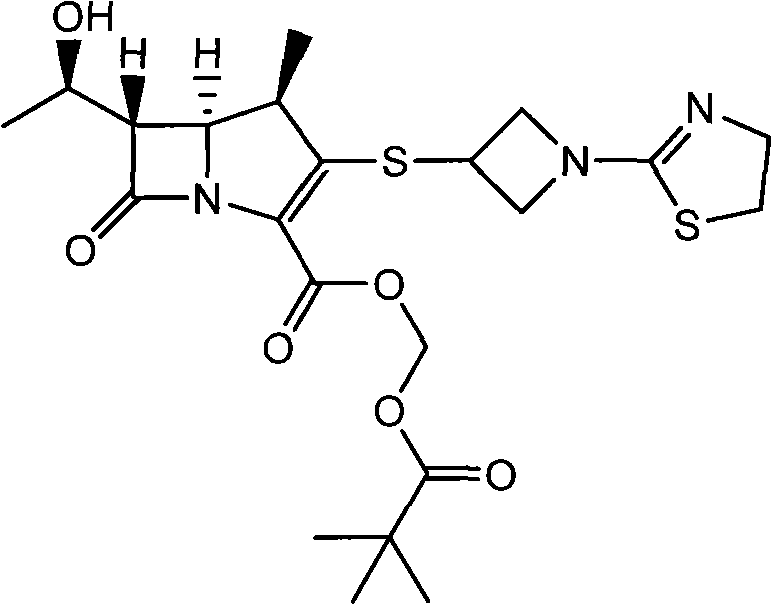
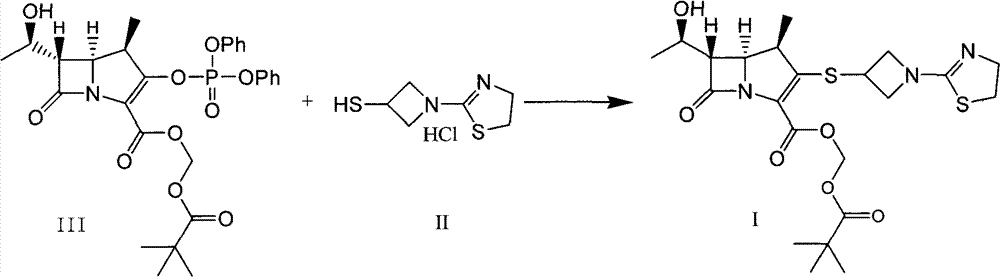
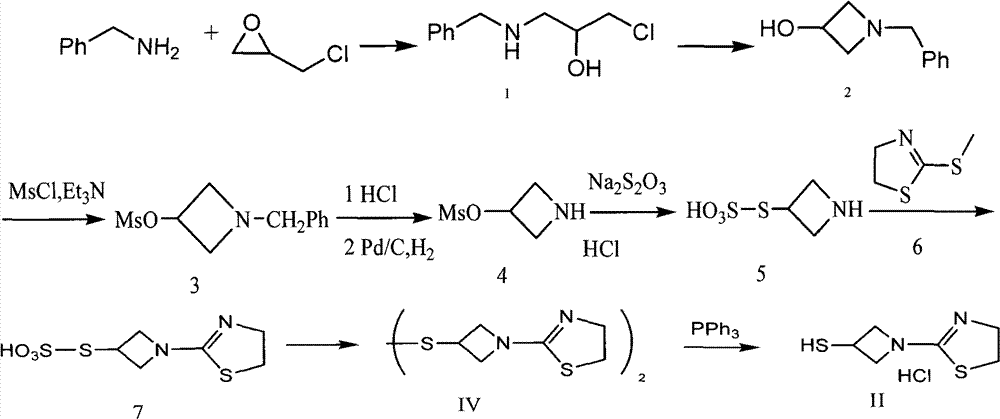
Preparation method for tebipenem pivoxil
A technology of tipipenem and pivate, which is applied in the field of preparation of tipipenem and pivate, can solve the problems of difficult preparation and storage, increase of production cost, equipment corrosion, etc., and achieve simple operation and simple post-processing , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Under nitrogen protection, compound (IV) (20.8g, 0.06mol), tributylphosphine (24.6g) and water (2.16g) were added to 300mL acetonitrile, stirred at room temperature for 40min, and the reaction solution was cooled to -10°C , then added compound (III) (57.4 g, 0.10 mol) and diisopropylethylamine (20.9 mL, 0.12 mmol) dropwise, and reacted at -10°C for 4 h after the dropwise addition.
[0043] Add 500mL ethyl acetate and 200mL water, adjust the aqueous solution to an acidic pH of 4.6, separate the layers, take the water layer, adjust the alkaline pH of the water layer to 7.8, extract twice with ethyl acetate (600mL*2), wash the organic layer with water, and saturated salt Washed with water, dried over anhydrous magnesium sulfate, filtered and concentrated.
[0044] Add 100mL of ethyl acetate, heat to dissolve, add dropwise 100mL of n-hexane, slowly cool to below 0°C, stir at the same temperature for 2h, filter and dry to obtain 47.1g of the target compound (I), with a yield...
Embodiment 2
[0046] Under argon protection, compound (IV) (20.8g, 0.06mol), tributylphosphine (26.7g) and water (2.16g) were added to 500mL acetonitrile, stirred at room temperature for 50min, and the reaction solution was cooled to -10 °C, compound (III) (57.4 g, 0.10 mol) was added, triethylamine (10.1 g, 0.10 mmol) was added dropwise, and reaction was carried out at -10 °C for 7 h after the dropwise addition.
[0047] Add 400mL ethyl acetate and 200mL water, adjust the aqueous solution to an acidic pH of 4.6, separate the layers, take the water layer, adjust the alkaline pH of the water layer to 8.2, extract twice with ethyl acetate (500mL*2), wash the organic layer with water, and saturated salt Washed with water, dried over anhydrous magnesium sulfate, filtered and concentrated.
[0048] Add 200 mL of ethyl acetate, heat to dissolve, slowly cool to below 0°C, stir at the same temperature for 1 h, filter, and dry to obtain 45.8 g of the target compound (I), with a yield of 92.2% and a ...
Embodiment 3
[0050] Under nitrogen protection, compound (IV) (20.8g, 0.06mol), tributylphosphine (30.3g) and water (2.16g) were added to 1000mL acetonitrile, stirred at room temperature for 20min, and the reaction solution was cooled to -10°C , then added compound (III) (57.4 g, 0.10 mol) and tetramethylguanidine (23.6 g, 0.20 mmol) dropwise, and reacted at -10°C for 5 h after the dropwise addition.
[0051] Add 500mL of ethyl acetate and 250mL of water, adjust the aqueous solution to an acidic pH of 3.9, separate the layers, take the water layer, adjust the alkaline pH of the water layer to 8.0, extract twice with ethyl acetate (550mL*2), wash the organic layer with water, and wash with saturated salt Washed with water, dried over anhydrous magnesium sulfate, filtered and concentrated.
[0052] Add 100mL of ethyl acetate, heat to dissolve, add dropwise 200mL of n-hexane, slowly cool to below 0°C, stir at the same temperature for 4h, filter and dry to obtain 44.8g of the target compound (I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com