Vaccine for preventing decayed tooth and preparation method thereof
A technology for vaccines and dental caries, which is applied in the field of medicine and biology, can solve problems such as application limitations, and achieve the effects of simple preparation methods, easy purification, and significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
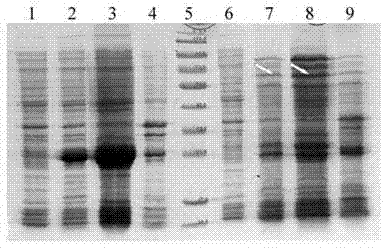
[0064] Embodiment 1, preparation prevents the antigenic protein of the vaccine of dental caries caused by S.Mutans infection
[0065] 1. PCR amplification of smu_862 DNA
[0066] 1. Primer design and synthesis
[0067] Primers were designed based on the "smu_862" nucleic acid sequence (812323..813030) in the S.mutans UA159 genome (GenBank: AE014133.1) published by GenBank. The 5' end of the upstream primer was introduced into the Sma I restriction site, and the 5' end of the downstream primer was introduced into NotI restriction site.
[0068] Primers were designed as follows:
[0069] smu_862-F 5'-TCC GCTATTTTGATAGTATTAGGCC-3'
[0070] Sma I restriction site
[0071] smu_862-R 5'-TTTTCCTTTT TCACCTCTTTTCATTAGCCTTA-3'
[0072] Not I restriction site
[0073] Primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0074] 2. PCR amplification of target DNA
[0075] 1) Preparation of template
[0076] S. mutans UA159 (purchased from American Type Cu...
Embodiment 2
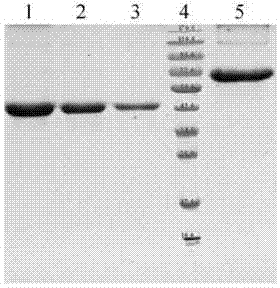
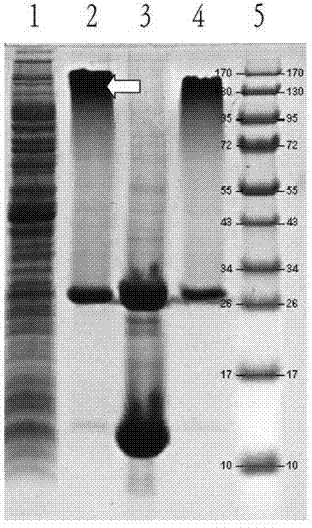
[0111] Example 2. Preparation of vaccines for preventing dental caries caused by S.mutans infection
[0112] 1. Preparation of LT S63K
[0113] (1) Whole gene synthesis LT S63K Gene
[0114] Synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. LT S63K The gene sequence is shown as sequence 4 in the sequence table, wherein the 1st to 6th positions from the 5' end are the Nde I restriction site sequence, the 7th to 60th are the LTA signal peptide sequence, and the 61st to 783rd are LT S63K A gene sequence, the 780th to 842nd position is the LTB signal peptide sequence, the 843rd to 842nd position is the LTB gene sequence, and the 1155th to 1160th position is the BamH I restriction site sequence (see sequence 4 in the sequence listing).
[0115] (2) Recombinant plasmid pET11c / LT S63K Construct
[0116] 1. Digestion
[0117] LT S63K The gene and pET11c plasmid (NOVAGEN, Code: 69438-3) were digested with restriction endonucleases Nde I (TaKaRa, Code: D1161A) and BamH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com