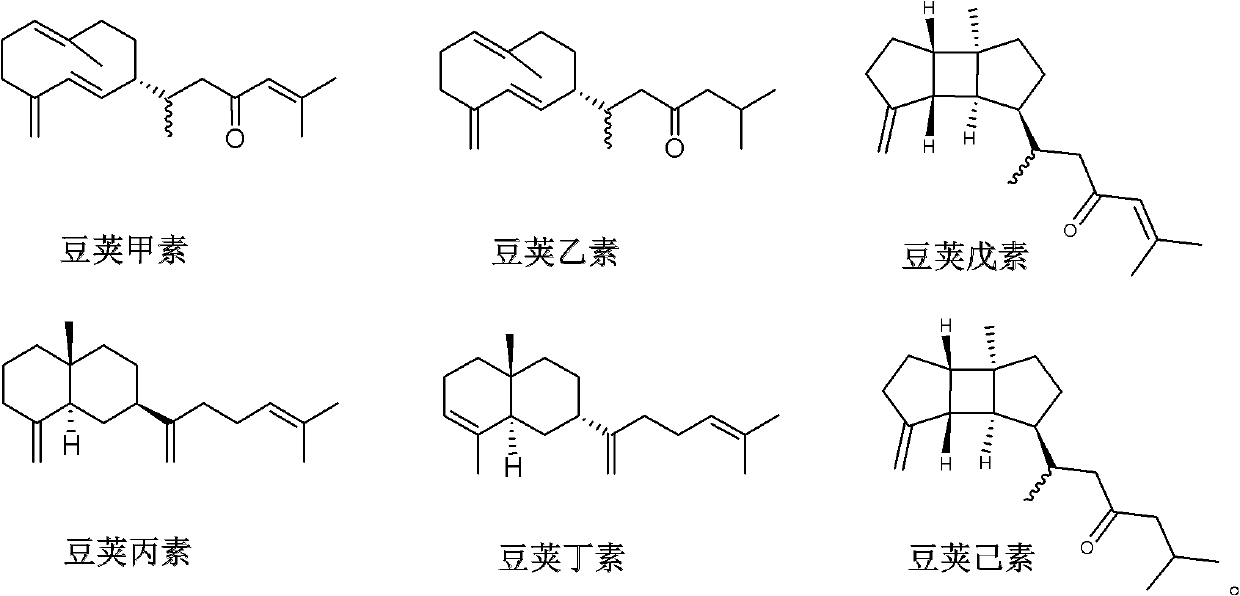
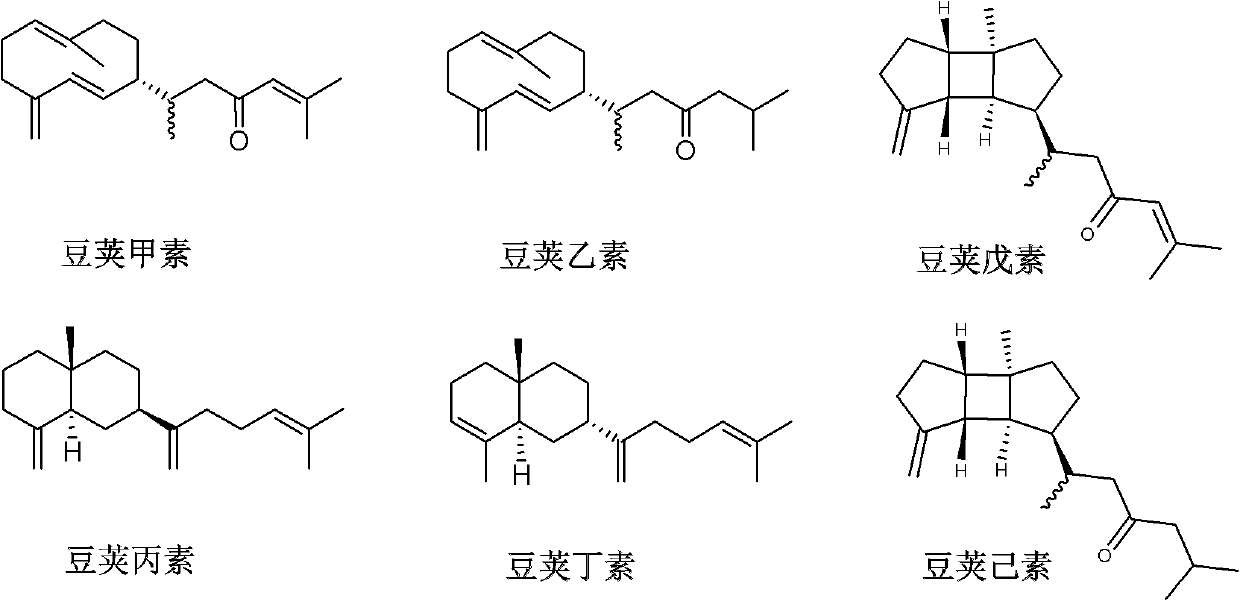
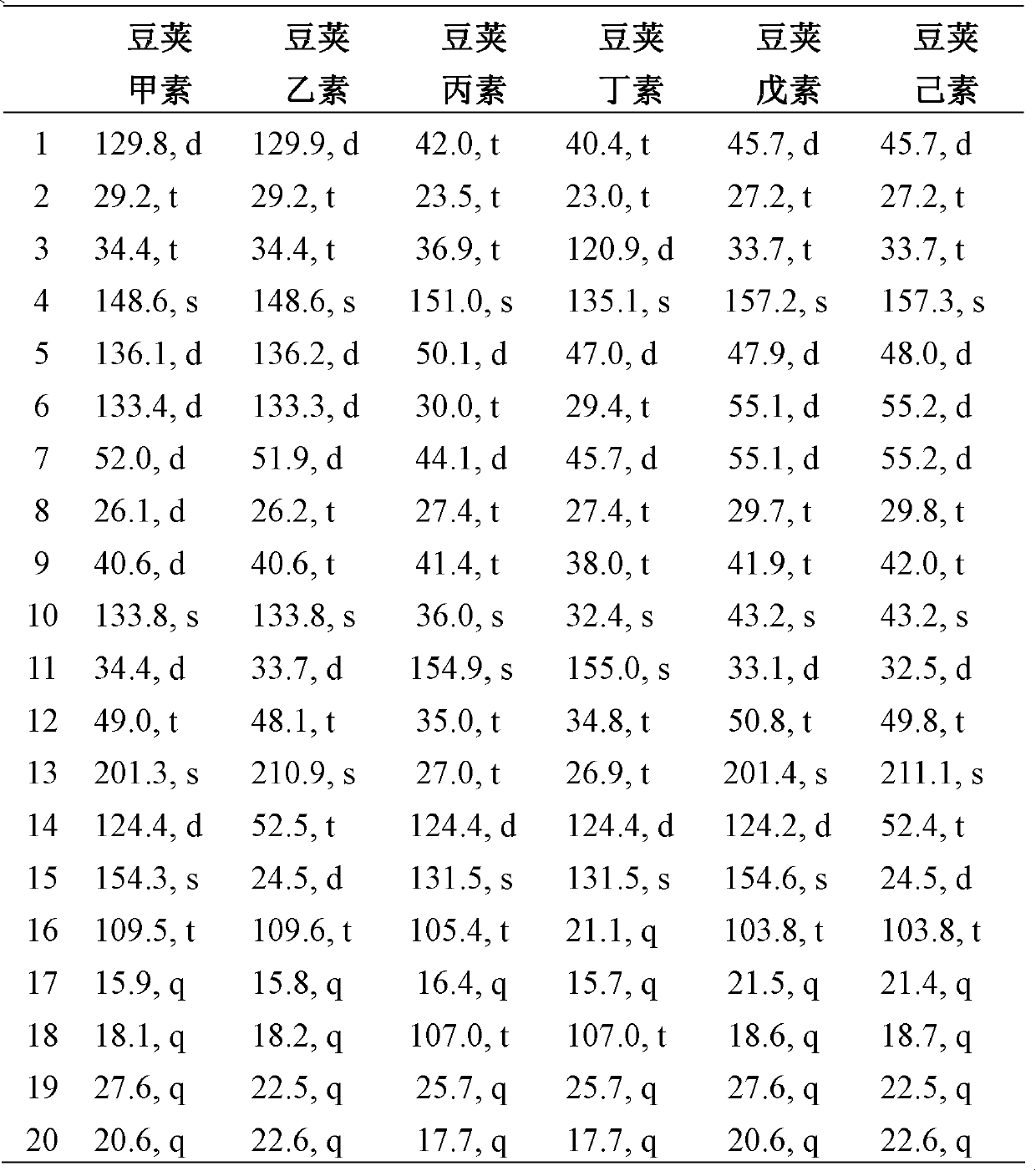
Diterpene compound Lobophytumin A, Lobophytumin B, Lobophytumin C, Lobophytumin D, Lobophytumin E and Lobophytumin F, and preparation method and application thereof to preparation of medicament
A kind of technology of legumes A and diterpenoids, applied in the field of medicine, can solve the problems of difficult drug candidate compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of diterpenoid legipin, second element, third element, butyl element, pentyl element and hexanol
[0026] (1) Extraction: the South China Sea pod soft coral Lobophytum cristatum with a dry weight of 210.0g is ultrasonically extracted 3 times with acetone (3000ml), the extracts are combined and concentrated under reduced pressure to obtain a crude extract, and the gained crude extract is mixed Suspended in 500ml of water. The suspension was extracted three times with ether (1000ml), and the obtained extracts were combined and concentrated under reduced pressure to obtain 5.4g of ether extract.
[0027] (2) Separation: 5.4 g of ether extract was subjected to 200-300 mesh silica gel column chromatography, and petroleum ether / ether (100:0→95:5→90:10→80:20→50:50→20:80→ 0:100) for gradient elution, the amount of eluent for each gradient was 1000ml. Among them, 625.3 mg of petroleum ether / diethyl ether (90:10) eluent concentrate was obtained. Th...
Embodiment 2
[0036] Embodiment 2: The PTP1B inhibitory activity experiment of diterpenoid legipin A, B, C, D, Penta and Hex
[0037] Test principle: p-Nitrophenyl phosphate (pNPP) is a soluble substrate of alkaline phosphatase, under the catalysis of human protein tyrosine phosphatase 1B (hPTP1B), it removes a phosphate group, Generate yellow soluble product p-Nitrophenol (p-Nitrophenol), this product has light absorption at 410nM place, can detect alkaline phosphatase by detecting the increase of light absorption at 410nM place (specifically refer to human source protein tyrosine Phosphatase 1B (hPTP1B)) activity.
[0038]
[0039] p-Nitrophenyl Phosphate p-Nitrophenol
[0040]Standard bioassay system: 10mM Tris.Cl (Tris (hydroxymethyl) aminomethane hydrochloride, namely tris (hydroxymethyl) aminomethane hydrochloride) (pH 7.6), 10mM pNPP, 2% DMSO (dimethyl sulphoxide, namely dimethyl sulfoxide) and 100 nM hPTP1B.
[0041] Test method: The protein tyrosine phosphatase PTP1B used for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com