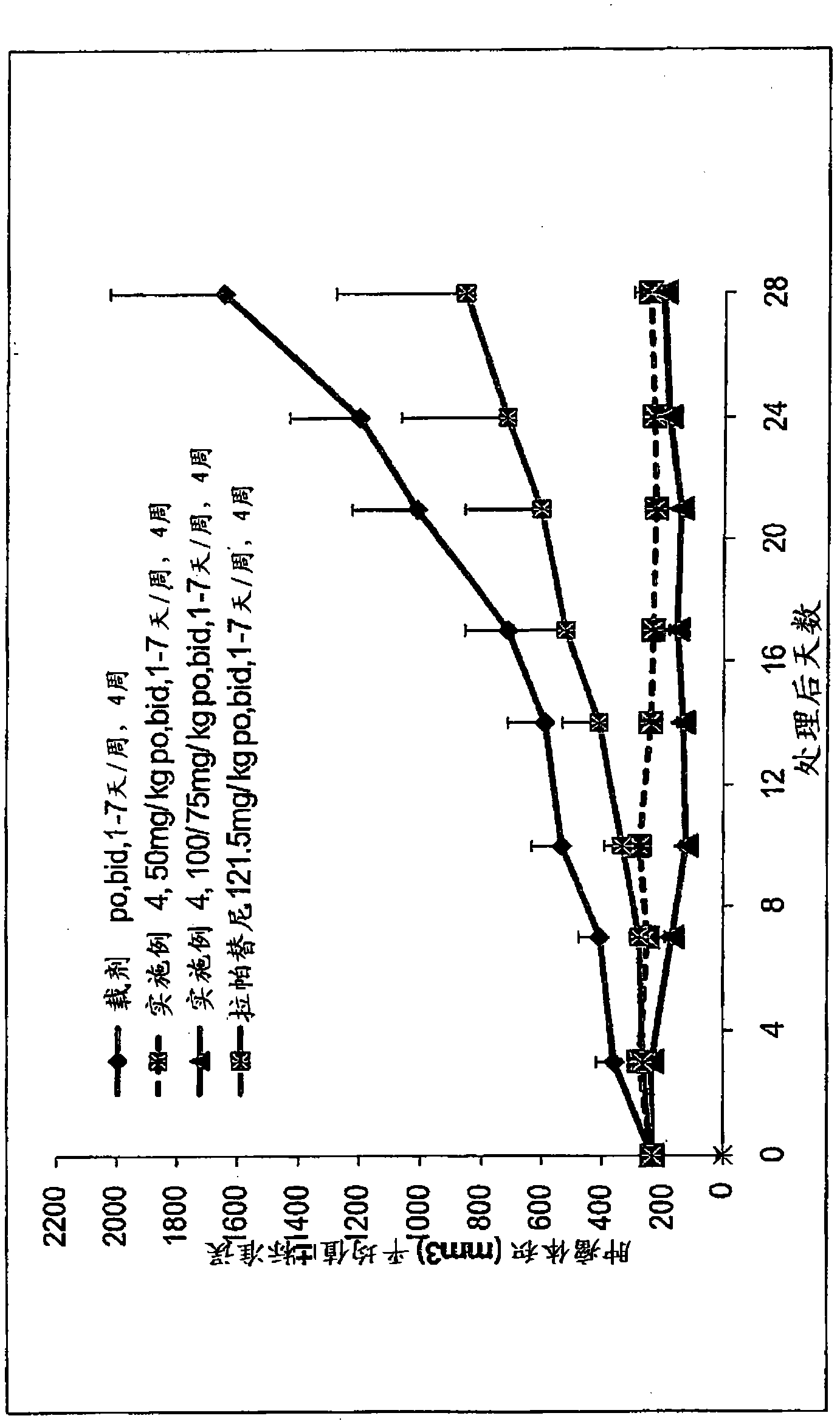
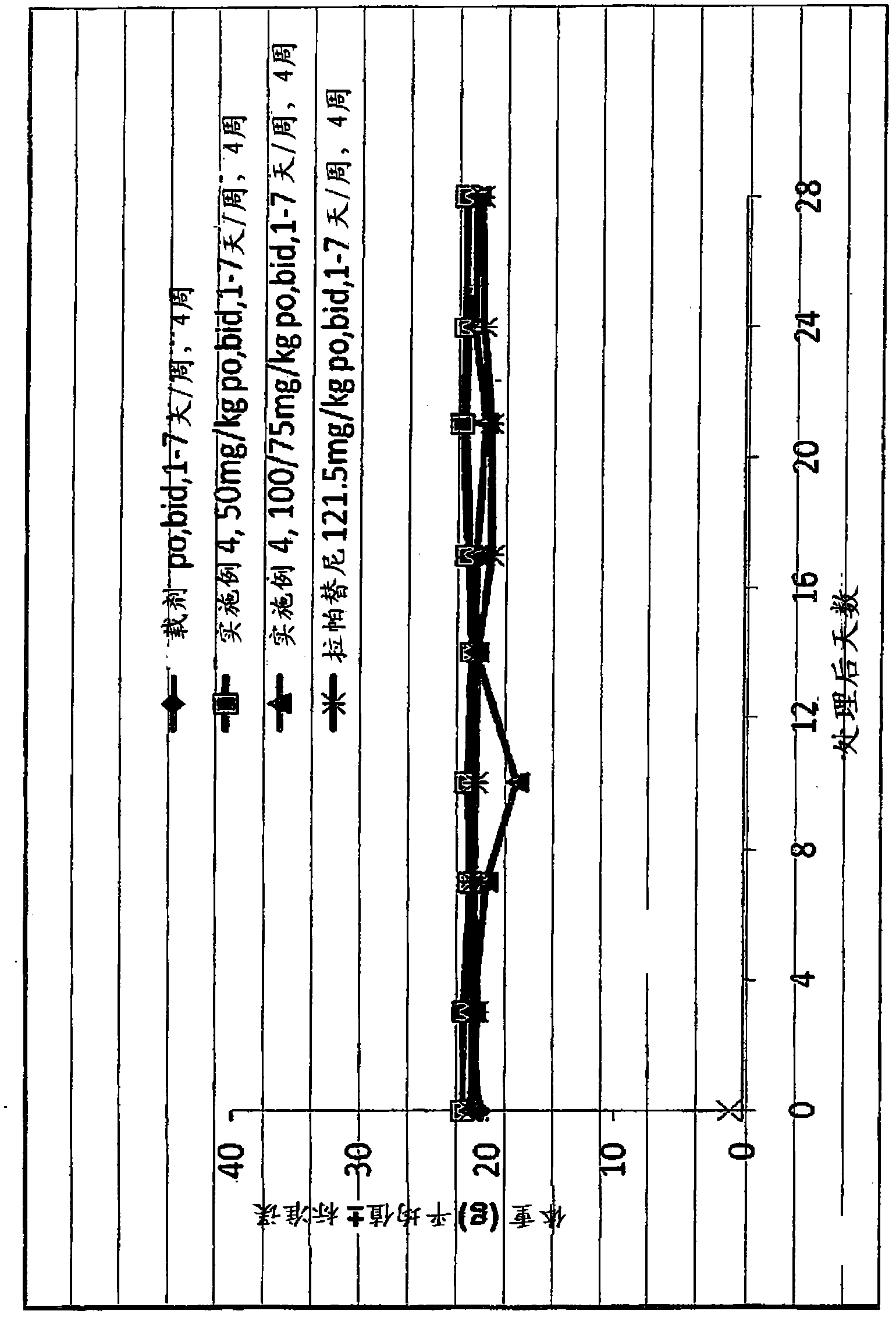
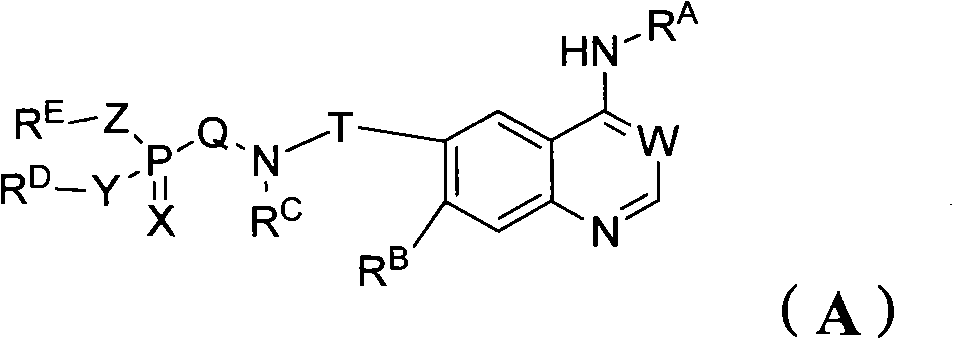
Phosphorus containing quinazoline compounds and methods of use
A kind of technology of compound and solvate, applied in the field of new quinazoline derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0283]
[0284] N-(3-chloro-4-(3-fluorobenzyloxy)phenyl)-6-(5-((4-methyl-1,4-azaphosphin-1-yl)methyl) furan-2-yl)quinazolin-4-amine
[0285] Step A: 1-Benzyl-4-methyl-1,4-azaphosphine-4-oxide (Compound 1.1)
[0286] To a vigorously stirred solution of methyl dichlorophosphoric acid (6.65 g, 50 mmol) in THF (100 mL) was added vinylmagnesium chloride (1.6 M in THF, 66 mL, 105 mmol) dropwise over a period of 30 minutes at -78 °C. After the addition was complete, the reaction mixture was warmed to 0° C. over 1 hour, and benzylamine (6.43 g, 60 mmol) and methanol (100 mL) were added thereto. The reaction mixture was then refluxed for 36 hours. After cooling to room temperature, the reaction mixture was poured into a separatory funnel containing saturated aqueous ammonium chloride (100 mL), ethyl acetate (200 mL) and water (100 mL). The organic layer was separated and the aqueous layer was extracted twice with dichloromethane (100 mL). The combined organic layer and extract w...
Embodiment 2
[0302]
[0303] 6-(5-((2-(Dimethylphosphino)ethylamino)methyl)furan-2-yl)-N-(4-(3-fluorobenzyloxy)-3-chlorobenzene base) quinazolin-4-amine
[0304] Step A: N-Benzyl-2-(dimethylphosphono)ethylamine (Compound 2.1)
[0305] To a vigorously stirred solution of dimethylphosphonyl chloride (6.65 g, 50 mmol) in THF (100 mL) was added vinylmagnesium chloride (1.6 M in THF, 33 mL, 52.5 mmol) dropwise over a period of 30 minutes at -78 °C . After the addition was complete, the reaction mixture was warmed to 0° C. over 1 hour, and benzylamine (6.43 g, 60 mmol) and methanol (100 mL) were added thereto. The reaction mixture was then refluxed for 36 hours. After cooling to room temperature, the solvent was removed and the residue was poured into a separating funnel containing ethyl acetate (200 mL) and water (100 mL). The organic layer was separated and the aqueous layer was extracted twice with dichloromethane (100 mL). The combined organic layer and extract were dried over anhydr...
Embodiment 3
[0312]
[0313] 6-(5-(((dimethylphosphono)methylamino)methyl)furan-2-yl)-N-(4-(3-fluorobenzyloxy)-3-chlorophenyl) Quinazolin-4-amine
[0314] 5-(4-(4-(3-fluorobenzyloxy)-3-chlorophenylamino)quinazolin-6-yl)furan-2-aldehyde (compound 1.7) and (dimethylphosphine Acyl)methylamine (Preparation: Maier, Ludwig Phosphorus, Sulfur and Silicon and the Related Elements, 53(1-4), 43-67; 1990) was suspended in dichloromethane (20 mL). Acetic acid (3 drops) and sodium triacetoxyborohydride (2 eq.) were then added sequentially at room temperature. The mixture was stirred at room temperature until the starting material disappeared. The reaction was quenched with 2N aqueous NaOH and extracted with dichloromethane. The combined organic layer and extract were dried over anhydrous magnesium sulfate, filtered and concentrated. The residue was purified by flash chromatography eluting with 20% methanol in dichloromethane to afford the desired product as a yellow crystalline solid.
[0315] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com