Competitive latex-particle-enhanced immunoturbidimetric assay kit for C-peptide and preparation method thereof
A detection kit and latex particle technology, applied in the biological field, can solve the problems of long detection time and high difficulty in detecting C-peptide by the sandwich method, and achieve the effect of simple detection method, low detection cost and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
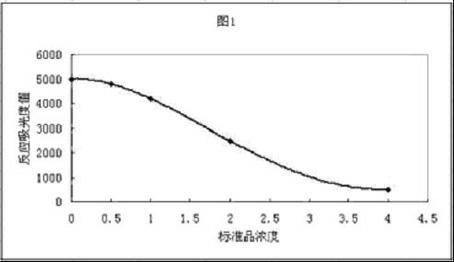
Image
Examples
Embodiment 1
[0031] The invention consists of latex particles and reaction buffer.
[0032] The latex particles are coated with the following artificially synthesized amino acid sequence: EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ-X; X consists of 2-4 K. The reaction buffer contains monoclonal antibody against EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ epitope. The EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ is the existing amino acid sequence.
[0033] The artificially synthesized amino acid sequence: EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ-X was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. The monoclonal antibody against EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ epitope was provided by Abcam.
[0034] (a) The preparation method of the latex particles consists of the following steps:
[0035] (a1) Use EDC and S-NHS to activate the latex particles, the activation time is 15 minutes, the activated material is centrifuged at 22000rpm for 10 minutes, the supernatant is removed, and the HEPES buffer is used to redissolve;
[00...
Embodiment 2
[0048] The difference between this example and Example 1 is that the artificially synthesized amino acid sequences coated with latex particles are different; meanwhile, the final concentration of the monoclonal antibody against the EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ epitope used in this example is 0.05 mg / ml. The artificially synthesized amino acid sequence used in this example is: EAEDLQVGQVELGGGPGAGSLQPLALEGSLQKKK. The test results are shown in Table 1.
Embodiment 3
[0050] The difference between this example and Example 1 is that the artificially synthesized amino acid sequences coated with latex particles are different; meanwhile, the final concentration of the monoclonal antibody against the EAEDLQVGQVELGGGPGAGSLQPLALEGSLQ epitope used in this example is 0.1 mg / ml. The artificially synthesized amino acid sequence used in this example is: EAEDLQVGQVELGGGPGAGSLQPLALEGSLQKKKK. The test results are shown in Table 1.
[0051] Table 1
[0052] sample Example 1 Example 2 Example 3 Controlled experiment 1 0.121 0.128 0.129 0.132 2 0.580 0.591 0.592 0.610 3 0.383 0.393 0.391 0.383 4 0.628 0.639 0.634 0.667 5 1.075 1.099 1.089 1.113 6 0.945 0.968 0.973 0.983 7 0.239 0.245 0.248 0.251 8 0.101 0.111 0.114 0.114 9 0.745 0.762 0.777 0.790
[0053] Through the above table 1, it can be effectively shown that the content (nmol / l) of C peptide in the sample can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com