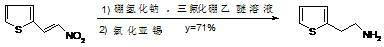Synthetic method of 2-thiofuran ethylamine
A technology of thienylethylamine and its synthetic method, applied in the direction of organic chemistry, etc.
Inactive Publication Date: 2012-09-12
TAIYUAN UNIV OF TECH
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0013] The technical problem to be solved by the present invention is to solve the problem of low yield and too long reaction time in the prior art, and then provide a high yield and short reaction time , environmental friendliness, be suitable for the synthetic method of the 2-thienylethylamine of suitability for industrialized production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0022] Example 2
Embodiment 2
[0024] Example 3
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a synthetic method of 2-thiofuran ethylamine. The method comprises the following steps of: cooling sodium borohydride and tetrahydrofuran, dropwise adding a boron trifluoride diethyl ether solution in a nitrogen atmosphere, and preserving heat for continually reacting; adding a solution consisting of 2-nitroethylene thiofuran and tetrahydrofuran into a reaction solution, reacting, heating, and continually reacting; and adding a hydrazine hydrate and RaneyNi, performing a reflux reaction, cooling, extracting with methylene dichloride, drying and distilling to obtain 2-thiofuran ethylamine. The method has the characteristics of high yield, low production cost, short reaction time, environmental friendliness and the like, and has industrial application prospect.
Description
technical field [0001] The invention relates to a method for synthesizing 2-thiopheneethylamine, in particular to a method for synthesizing 2-thiopheneethylamine through reduction using 2-nitroethylenethiophene as a raw material. Background technique [0002] 2-Thienylethylamine is an important chemical intermediate, which can be used to synthesize a variety of compounds, such as hypolipidemic drugs, platelet coagulation inhibitors, cardiovascular relaxants, 5-lipoxygenase inhibitors, antibiotics, etc. Its structural formula is as follows: [0003] [0004] There are many ways to synthesize 2-thienylethylamine. Journal of Wuhan Institute of Chemical Technology, 2002,24 (3): 14-16 publicly reported by 2-nitroethylenethiophene through sodium borohydride, stannous chloride reduction double bond and nitro to obtain 2-thiophene ethylamine respectively, its reaction The equation is: [0005] [0006] The amount of the reducing agent stannous chloride used in this method i...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D333/20
Inventor 苗茂谦邢俊德张照昱贾小棐
Owner TAIYUAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com