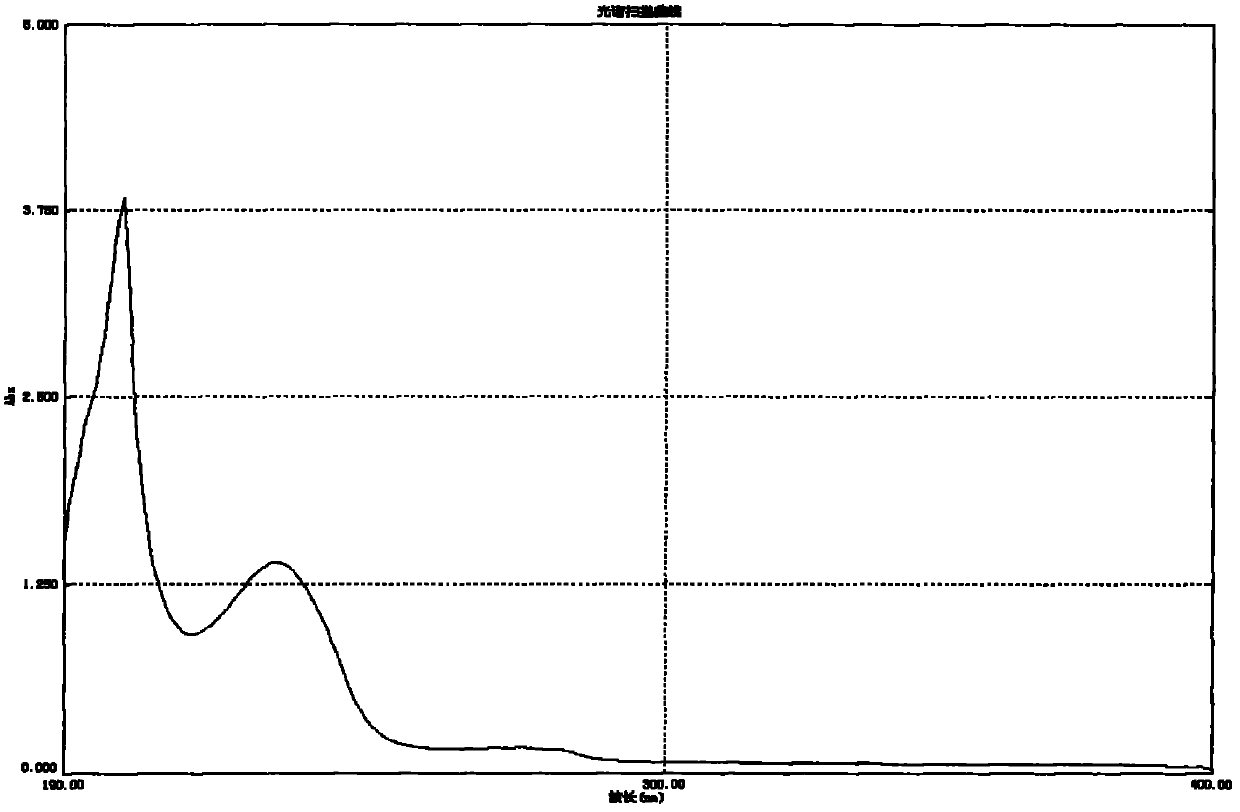
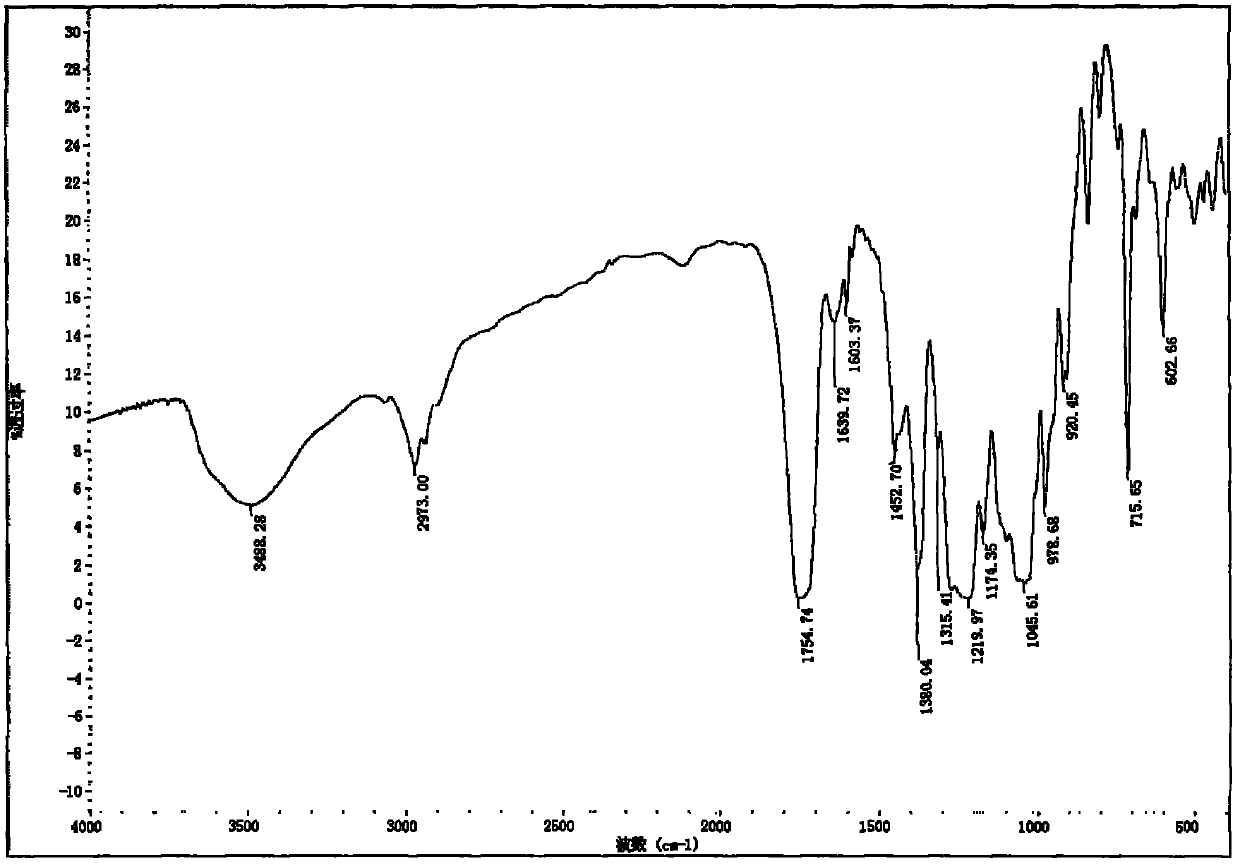
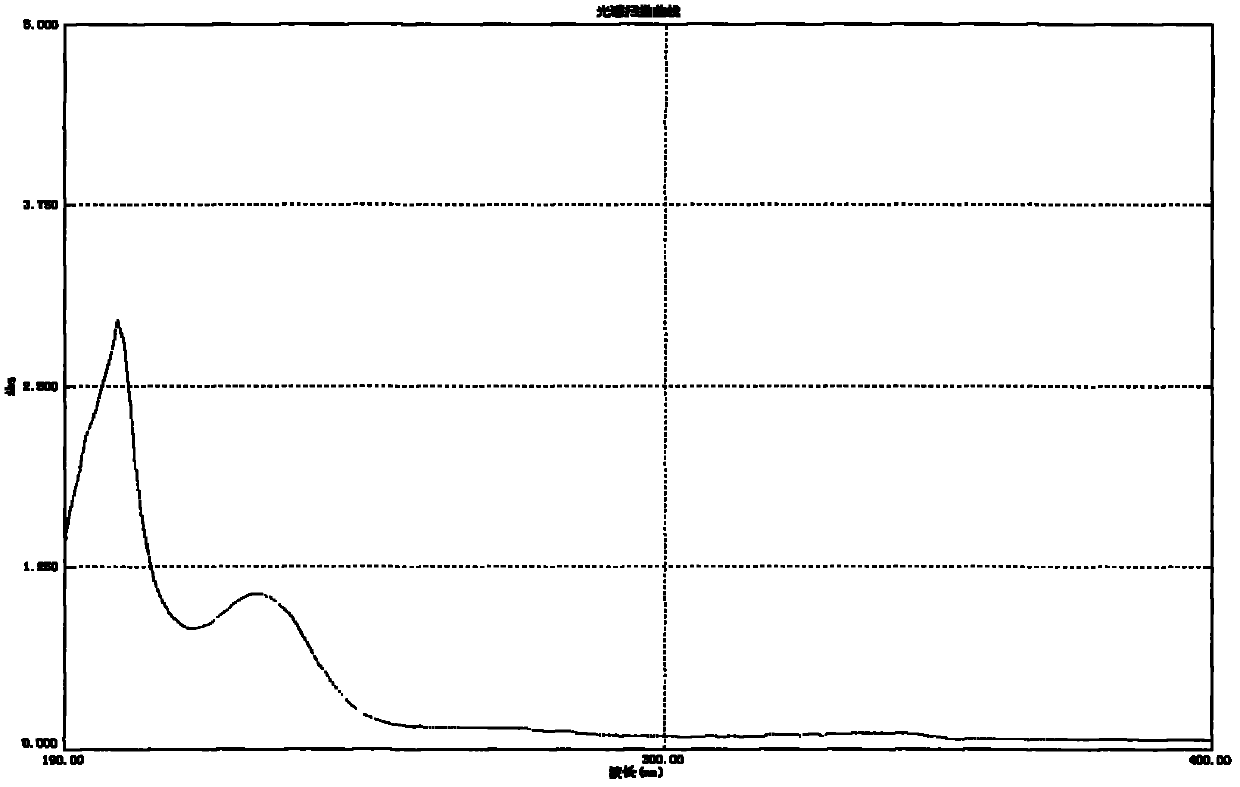
Acylated glycoside compounds as well as production method and application thereof
A technology of acylated glycosides and compounds, applied in the field of acylated glycosides compounds, can solve the problems of low bioavailability, poor gastrointestinal absorption of paeoniflorin and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the synthesis of paeoniflorin tosylate
[0033] Accurately weigh 2g of paeoniflorin and dissolve it in pyridine (10ml), slowly add dichloromethane solution (5ml) containing p-toluenesulfonyl chloride (3.5g, 18mmol) dropwise in an ice-water bath, stir and react for 10h, then wash with 500ml of water Twice, and then extracted with 200ml ethyl acetate, the organic layer was taken and dried with an appropriate amount of anhydrous sodium sulfate. Let stand for a period of time, filter, and spin evaporate. Finally, it was recrystallized from absolute ethanol to obtain 0.51 g of a solid crude product in the form of light yellow powder, with a yield of 25.5%.
Embodiment 2
[0034] Embodiment 2, the purification of paeoniflorin tosylate
[0035] Accurately weigh 0.5 g of the crude product of paeoniflorin p-tosylate, dissolve with a small amount of eluent, and slowly load the sample on the silica gel column with a dropper (120 g of silica gel for column chromatography, the silica gel column is glass column, wet packed). Elute with dichloromethane-methanol (15:1, V / V), the amount of eluent depends on the separation effect, while controlling the flow rate, generally 5.0ml min -1 . Time tracking and identification by TLC method, 0.32g of paeoniflorin p-tosylate was obtained, the yield was 64.0%.
Embodiment 3
[0036] Embodiment 3, the synthesis of paeonifloride glycoside tosylate
[0037] Accurately weigh 0.5 g of paeonifloride and dissolve it in pyridine (8 ml), slowly add dropwise a dichloromethane solution (5 ml) containing p-toluenesulfonyl chloride (1 g, 9 mmol) in an ice-water bath, stir and react for 10 h, then use 100ml of water was washed twice, and then extracted with 100ml of ethyl acetate, and the organic layer was dried with an appropriate amount of anhydrous sodium sulfate. Let stand for a period of time, filter, and spin evaporate. Finally, it was recrystallized from absolute ethanol to obtain a light yellow powder solid crude product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com