Chitosan-chitosan derivative nanosphere for loading indissoluble medicament, preparation method of nanosphere, and application of nanosphere serving as oral prepration
A technology of chitosan derivatives and insoluble drugs, which is applied in the field of pharmaceutical preparations, can solve the problems of easy escape of drugs, limited preparation methods, and reduced drug embedding rate, so as to achieve bioavailability, good cell and adhesion, The effect of good batch reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
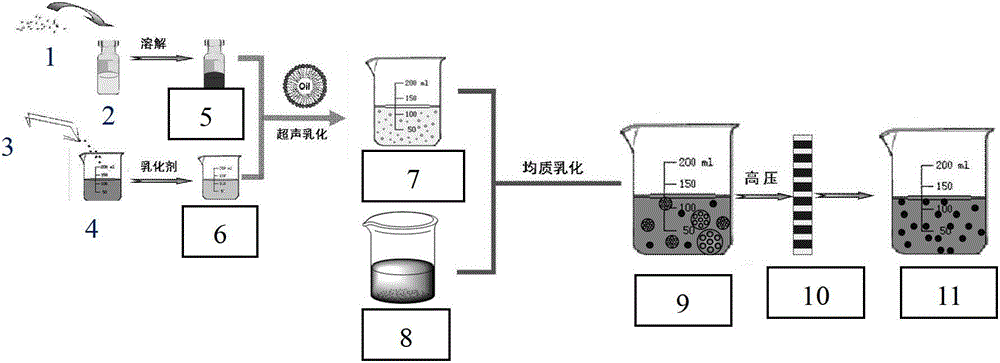
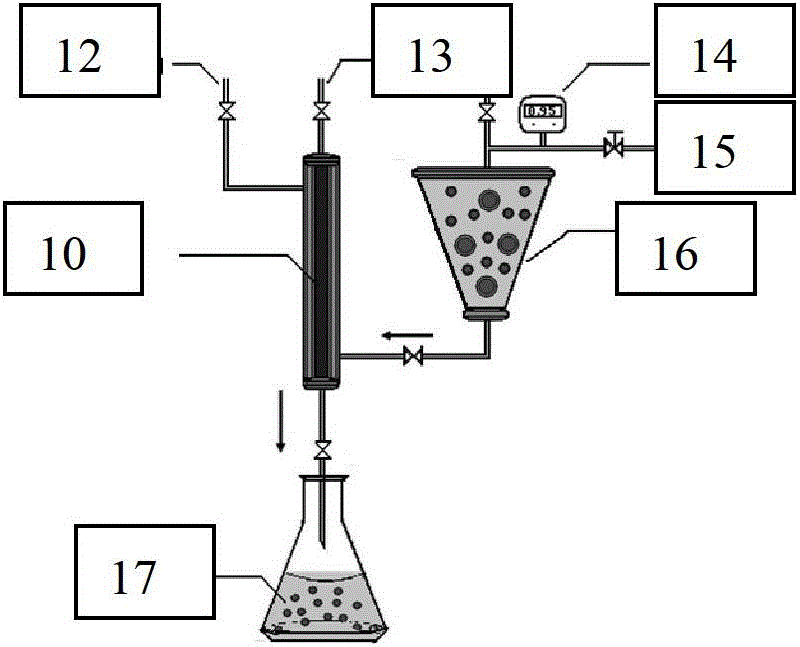
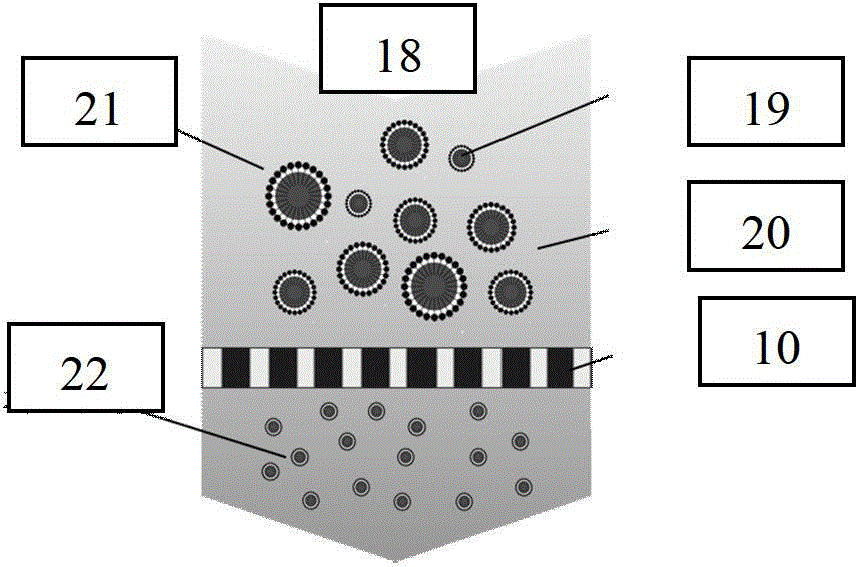
Image
Examples
Embodiment 1
[0099] Example 1: Preparation of chitosan-chitosan derivative nanospheres (HTCC-NP:PTX) loaded with poorly soluble drugs
[0100] In this implementation, paclitaxel was selected as the insoluble drug model, and quaternized chitosan was used as the chitosan derivative model. First, soak the hydrophilic membrane with a pore size of 0.5 μm in a mixed oil phase of liquid paraffin and petroleum ether (volume ratio 4:1) overnight or use an ultrasonic cleaner for 1 hour to fully wet the membrane pores with the oil phase. Accurately weigh a certain amount of paclitaxel powder, an insoluble drug, and dissolve it in dichloromethane to a concentration of 60 mg / mL, and use it as the internal oil phase for future use. Weigh a certain amount of chitosan and quaternized chitosan mixture (mass ratio is 1:2) and dissolve in 1% acetic acid aqueous solution to obtain chitosan-quaternized chitosan aqueous acetic acid solution, the concentration 1.0wt%, while adding 8% water phase emulsifier 35...
Embodiment 2
[0102] Example 2: Preparation of chitosan-chitosan derivative nanospheres loaded with different insoluble drugs
[0103] Carboxylated chitosan was selected as the chitosan derivative model. Firstly, the hydrophilic membrane with a pore size of 1.0 μm was soaked overnight in a mixed oil phase of soybean oil and sunflower oil at a volume ratio of 2:1, or ultrasonic cleaner was used for 1 hour to fully wet the membrane pores by the oil phase. Accurately weigh a certain amount of O-(chloroacetylcarbamoyl) fumagillin alcohol, nimodipine, oleanolic acid, and tanshinone IIA powder and dissolve them in the inner oil phase of chloroform, with a concentration of 10 mg / mL , 30mg / mL, 40mg / mL, 80mg / mL, as the internal oil phase for later use. Take a certain amount of chitosan-carboxylated chitosan (mass ratio is 10:1) and dissolve in 1% citric acid aqueous solution to obtain chitosan-carboxylated chitosan aqueous solution, its concentration is 2.0wt%, At the same time add 5% water phase ...
Embodiment 3
[0104] Example 3: Preparation of chitosan-chitosan derivative nanospheres with different pore sizes loaded with insoluble drugs
[0105] In this example, paclitaxel was selected as the insoluble drug model, and thiolated chitosan was selected as the chitosan derivative model. Firstly, the hydrophilic membrane with a pore size of 3.0 μm was soaked overnight or ultrasonicated for 3 hours in a mixed oil phase of liquid paraffin and cottonseed oil (volume ratio 1:2), so that the membrane pores were fully wetted by the oil phase. A certain amount of paclitaxel powder, an insoluble drug, was accurately weighed and dissolved in dichloromethane of the inner oil phase to a concentration of 80 mg / mL, and used as the inner oil phase for later use. The mixture of a certain amount of chitosan and quaternized chitosan was weighed and dissolved in 1% acetic acid aqueous solution, and the mass ratios of chitosan and thiolated chitosan were respectively determined as 10:1, 1:1 and 1:1. 10. Ob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com