Preparation method of irinotecan hydrochloride
A technology of irinotecan hydrochloride and irinotecan, which is applied in the direction of organic chemistry, can solve problems such as environmental pollution, hidden dangers in production safety, and increased reaction steps, and achieve the effects of easy transportation, safe use, and pollution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
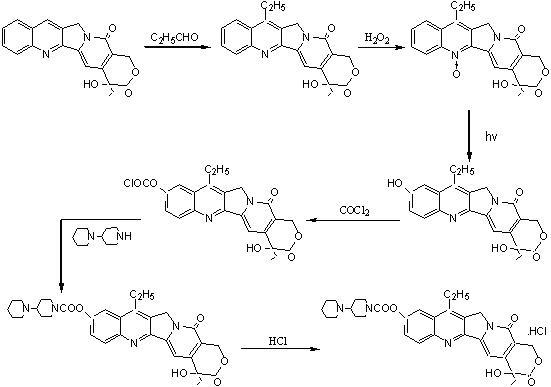
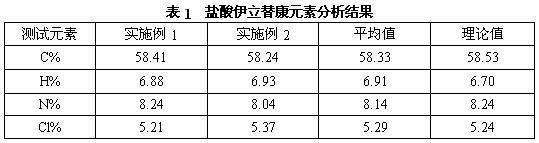
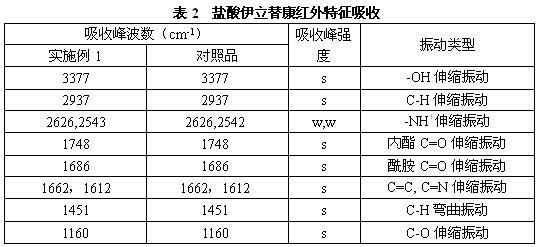
[0047] a) Add 4-piperidinyl piperidine (168 g, 1.00 mol), N, N-dimethylformamide (DMF) 900 mL, dimethyl carbonate ( 90 g, 1.00 mol) after the addition, control the temperature of the reaction solution at 60°C to 65°C and stir for 3 hours. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain a viscous oily substance, weighing 243g, with a yield of 100%. The intermediates in this step did not need to be purified in full to continue.
[0048] b) In a reaction flask equipped with stirring, thermometer, and condensing device, add 4-piperidinylpiperidine methyl carbonate (243 g, 1.00mol) prepared in the previous step, 1000 mL of chloroform, 7-ethyl- After the 10-hydroxycamptothecin (263 g, 0.67 mol) was stirred evenly, the temperature of the reaction solution was controlled at 45°C to 50°C and stirred for 6 hours. After the reaction was completed, 500 mL of water was added, and the chloroform layer was separated. The water layer was extra...
Embodiment 2
[0051] a) Add 4-piperidinylpiperidine (168g, 1.00 mol), acetonitrile 900mL, and dimethyl carbonate (90g, 1.00 mol) into a reaction flask equipped with stirring, thermometer, and condensing device, then control the reaction The liquid temperature was 60°C to 65°C and stirred for 3 hours. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain a viscous oily substance, weighing 240 g, with a yield of 100%. The intermediates of this step did not need to be purified in full to continue.
[0052] b) Add 4-piperidinylpiperidine methyl carbonate (240g, 1.00mol) prepared in the previous step reaction, 800 mL of dichloromethane, 7-ethyl -10-Hydroxycamptothecin (196 g, 0.50 mol) was stirred evenly, and the temperature of the reaction solution was controlled at 40°C to 45°C and stirred for 6 hours. Methane was extracted once, the combined dichloromethane layers were concentrated to dryness under reduced pressure, then 1150 mL of absolute ethanol...
Embodiment 3
[0055] a) Add 4-piperidinylpiperidine (168g, 1.00 mol), hexamethylphosphoramide 900mL, and dimethyl carbonate (90g, 1.00 mol) into a reaction flask equipped with stirring, thermometer, and condensing device After that, control the temperature of the reaction solution at 60° C. to 65° C. and stir for 3 hours. After the reaction was completed, it was concentrated to dryness under reduced pressure to obtain a viscous oily substance, weighing 240 g, with a yield of 100%. The intermediates of this step did not need to be purified in full to continue.
[0056] b) In a reaction flask equipped with stirring, thermometer, and condensing device, add 4-piperidinylpiperidine methyl carbonate (240g, 1.00mol) prepared in the previous step, petroleum ether 1000 mL, 7-ethyl- After stirring 10-hydroxycamptothecin (263 g, 0.67 mol) evenly, control the temperature of the reaction solution at 45°C to 50°C and stir for 6 hours. After the reaction, add 500mL of water to separate the petroleum ether...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com