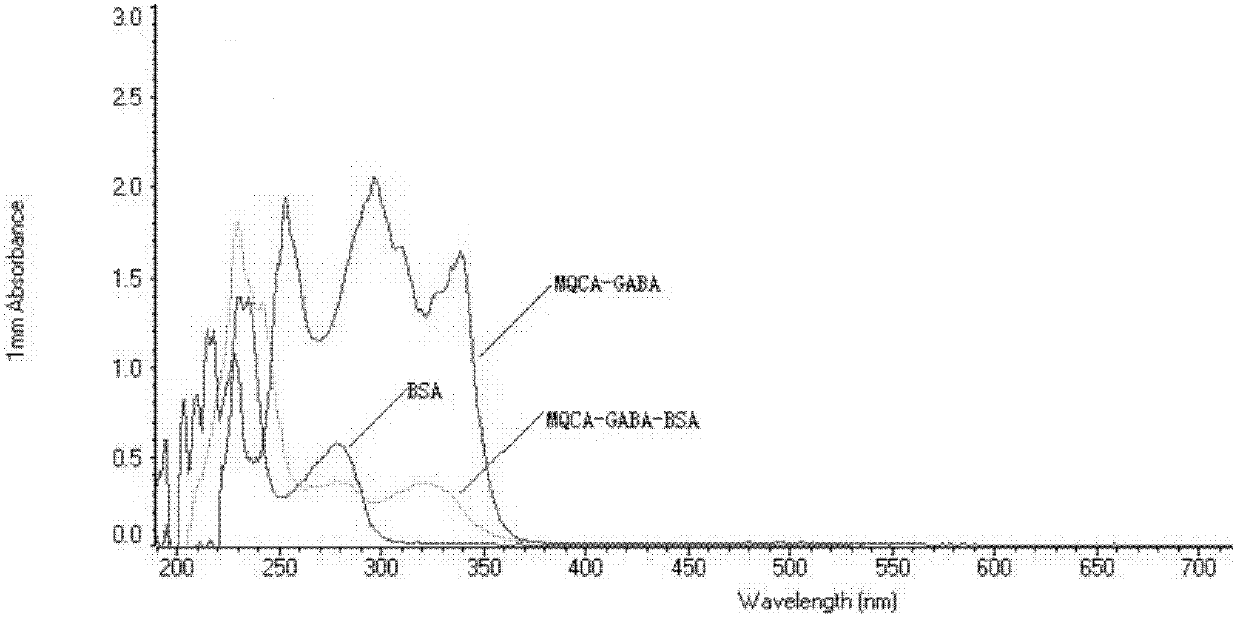
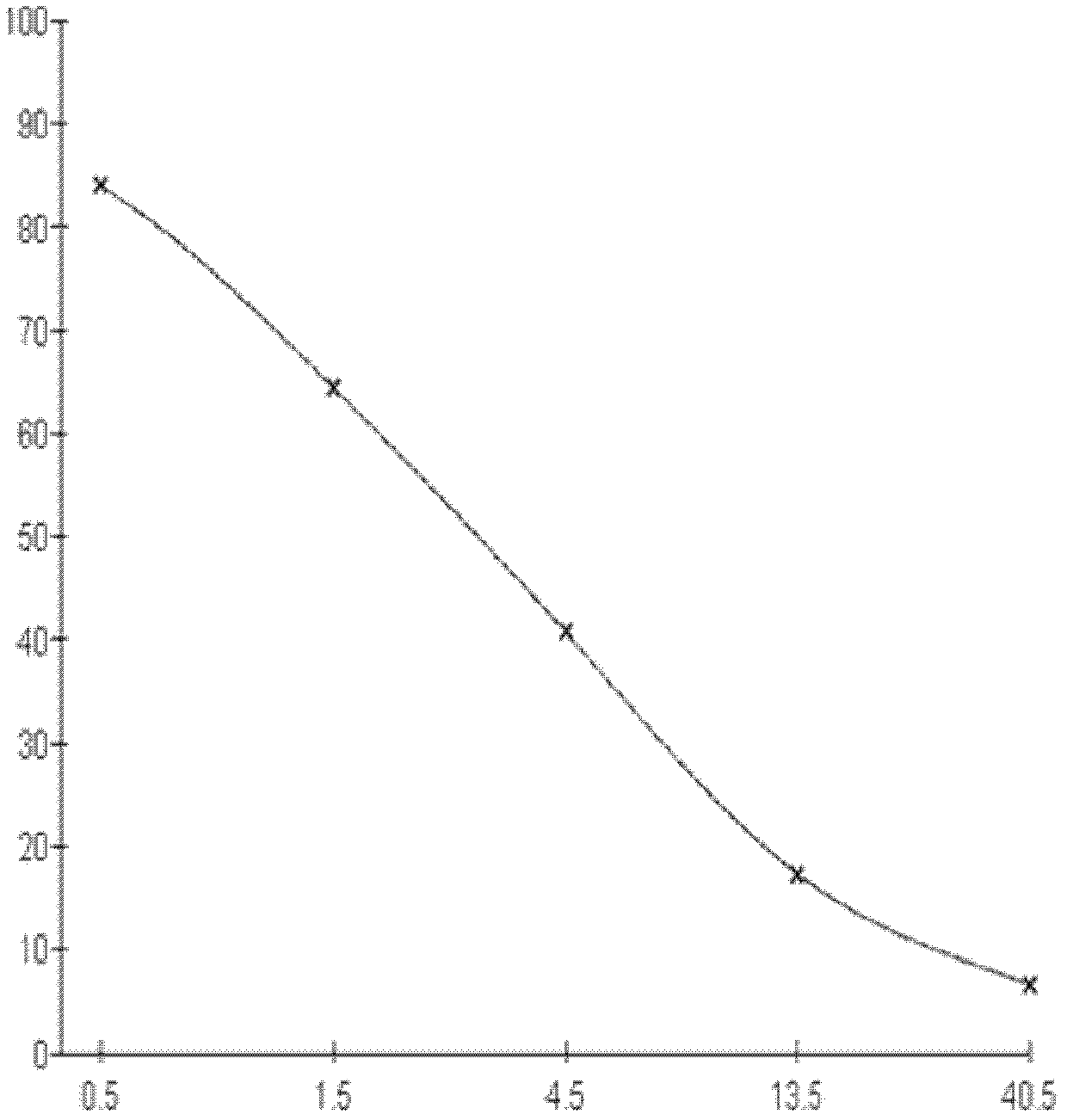
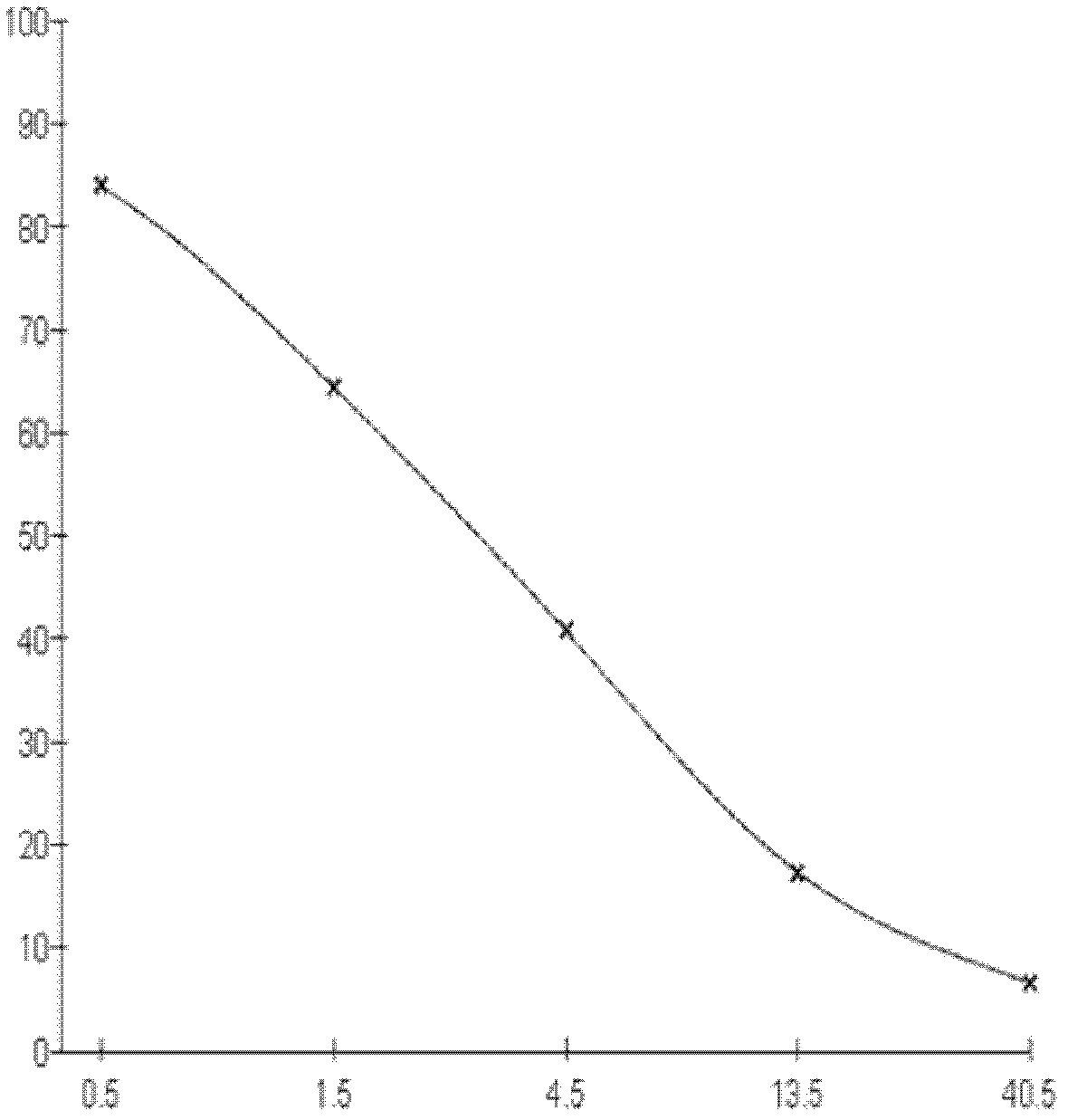
Kit for detecting 3-methyl-quinoxaline-2-carboxylic acid
A methylquinoxaline and kit technology, which is applied in the field of kits for detecting 3-methylquinoxaline-2-carboxylic acid, can solve photosensitivity and adrenal cortex damage, endanger human and animal health, cause mutagenesis, etc. problems, to achieve the effect of convenient and easy inspection methods, long shelf life and high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1, preparation 3-methylquinoxaline-2-carboxylic acid hapten
[0041] One, the preparation of 3-methylquinoxaline-2-carboxylic acid hapten
[0042] 1. Weigh 10 mg of 3-methylquinoxaline-2-carboxylic acid and place it in a 10mL reaction bottle; add an appropriate amount of acetone and a small amount of DMF to completely dissolve it (acetone is used as a reaction system to dissolve 3-methylquinoxaline-2 -Carboxylic acid, the role of DMF is to promote dissolution), add 10 μL of thionyl chloride (acting as activated carboxyl group), heat and reflux for 1 h; then add 0.5 ml of n-hexane and blow it with nitrogen until the volume is constant, then add 0.5 ml of n-hexane and blow it with nitrogen Blow until the volume is constant, then add 0.5 ml of n-hexane and blow with nitrogen until the volume is constant to obtain solution I.
[0043] 2. Weigh 20mg of γ-aminobutyric acid, dissolve it in 1ml of 2mol / L KOH aqueous solution (as a reaction system to dissolve γ-amino...
Embodiment 2
[0050]Preparation and characterization of embodiment 2, 3-methylquinoxaline-2-carboxylic acid artificial antigen
[0051] 1. Synthesis and characterization of 3-methylquinoxaline-2-carboxylic acid immunogen
[0052] 1. Preparation of 3-methylquinoxaline-2-carboxylic acid immunogen
[0053] (1) Dissolve 10 mg of the compound shown in formula (I) prepared in Example 1 in 2 mL of N, N'-dimethylamide, add 10 mg of N-hydroxysuccinimide and 10 mg of 1-ethyl-(3- Dimethylaminopropyl) carbodiimide hydrochloride, magnetically stirred at room temperature for 2 h to obtain solution III.
[0054] (2) Add 30mg of bovine serum albumin into 2mL of PBS buffer solution and fully dissolve to obtain solution IV.
[0055] (3) Slowly add solution III to solution IV, stir slowly for 24 hours, put it into a dialysis bag, dialyze in normal saline at 4°C for 72h (change the water 6 times in the middle), then centrifuge at 8000rmp for 30min at 4°C, and take The supernatant, that is, the 3-methylquino...
Embodiment 3
[0069] Preparation of embodiment 3, 3-methylquinoxaline-2-carboxylic acid monoclonal antibody
[0070] Balb / c mice: purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.;
[0071] SP2 / 0 myeloma cells: purchased from Sigma-Aldrich Company, the product catalog number is 08060101.
[0072] 1. Animal immunity
[0073] The MQCA-BSA solution prepared in Example 2 was used to immunize Balb / c mice, and each mouse was immunized with 100 μg MQCA-BSA once, for a total of 4 times with an interval of two weeks between each time. Injection, the last three immunizations were intraperitoneal injection.
[0074] 2. Cell fusion and cloning
[0075] 1. Three days after the fourth immunization, splenocytes were collected and fused with SP2 / 0 myeloma cells at a ratio of 5:1 (quantity ratio). Cell supernatants were measured by indirect competitive ELISA, and positive wells were screened.
[0076] 2. Cloning the positive wells by using the limiting dilution method to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com