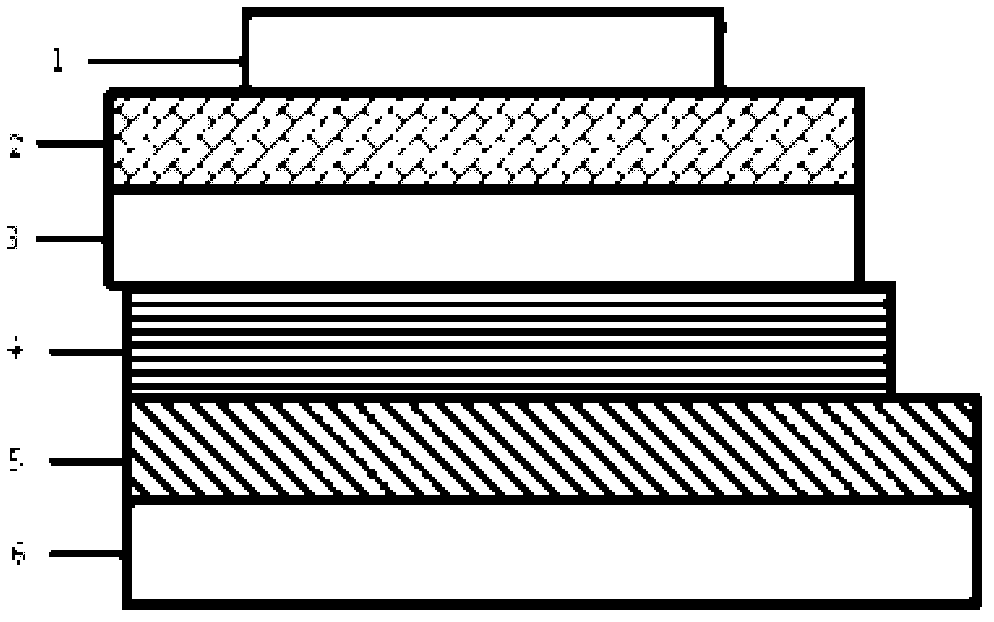
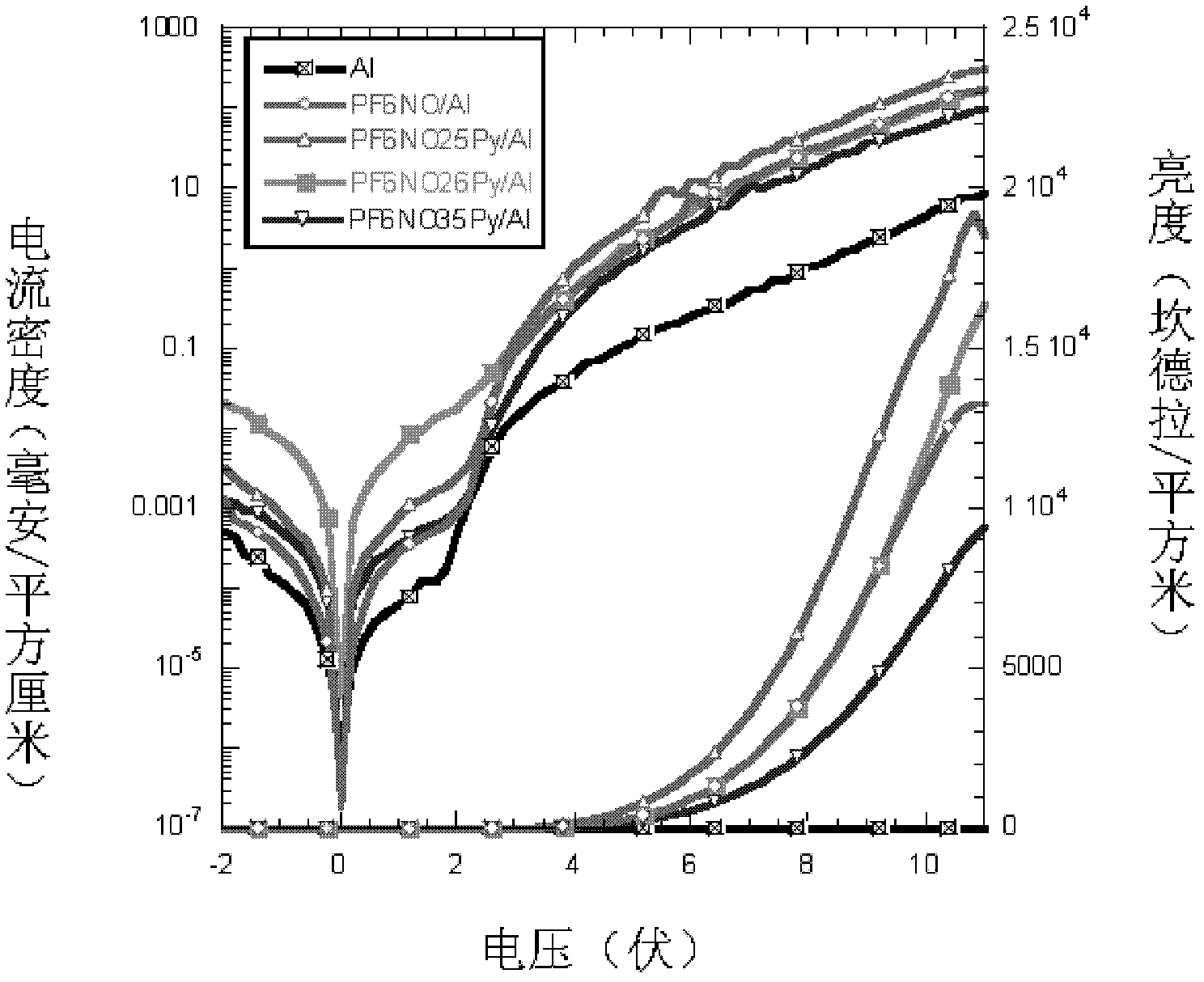
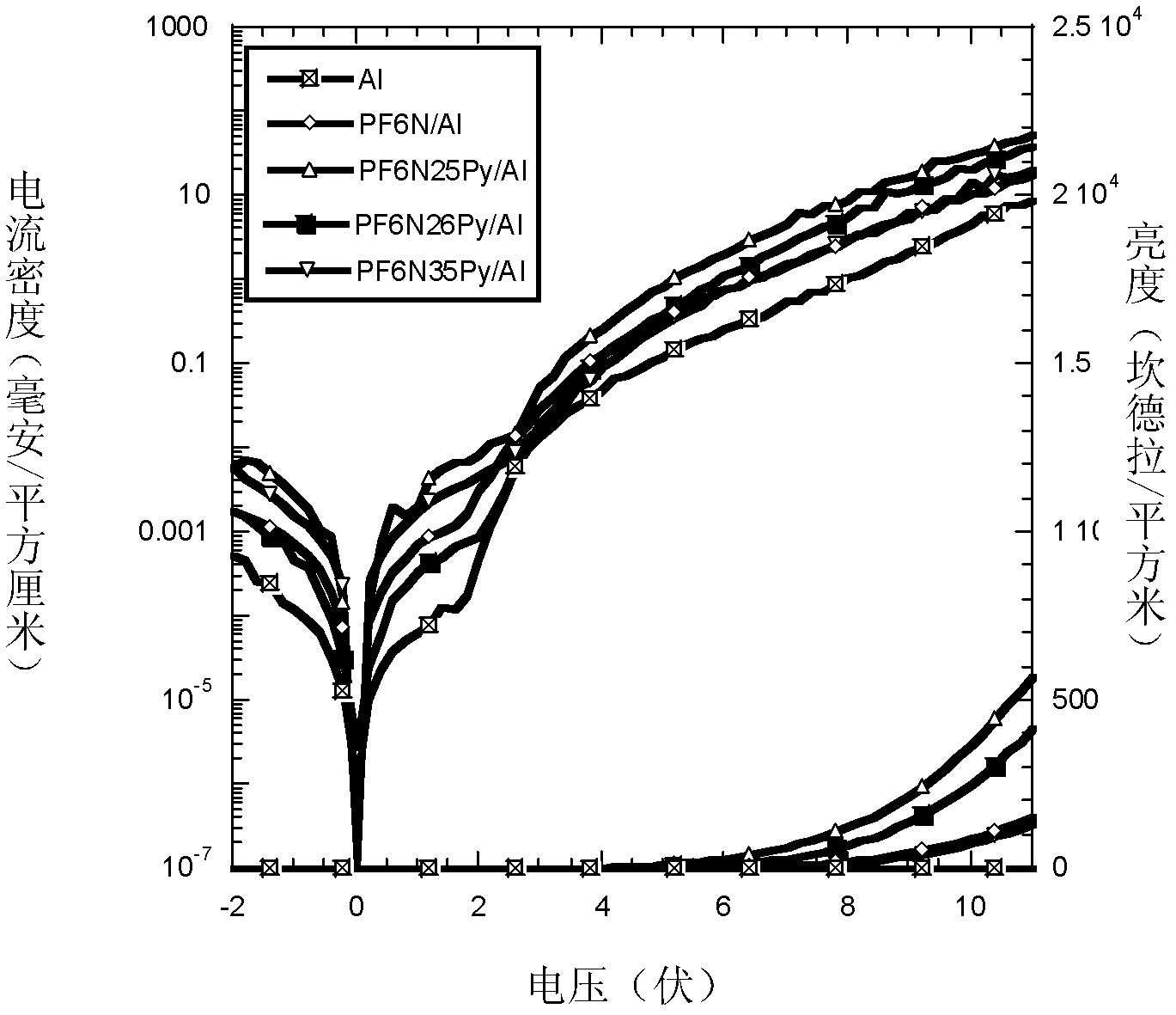
Conjugated polymer photoelectric material containing amine oxide groups and application thereof
A technology of conjugated polymers and amine oxides, which is applied in luminescent materials, photovoltaic power generation, circuits, etc., can solve the problems of response speed conjugated polyelectrolyte charge mobility ions, affecting long-term stability of devices, etc., to achieve excellent electronic performance Effect of Injection/Transport Performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: The synthetic route of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amine oxide)fluorene]} (PF6NO) is as follows:
[0031]
[0032] (1) Monomers 1 and 2 are prepared according to the method disclosed in the literature [Adv.Mater., 2011, 23, 1665];
[0033] (2) Preparation of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amino)fluorene]} (PF6N)
[0034] Monomer 2,7-bis(trimethylene borate)-9,9'-bis(N,N-diethylhexyl-6-amino)fluorene (728 mg, 1 mmol), monomer 2,7 -dibromo-9,9'-bis(N,N-diethylhexyl-6-amino)fluorene (634mg, 1mmol) and 10mg tetraphenylphosphorous palladium catalyst dissolved in 10ml toluene and 5ml tetrahydrofuran In the solvent, add 4ml of 2mol / L sodium carbonate aqueous solution, under the protection of argon, reflux reaction for 48 hours, then cool to room temperature, precipitate the reaction solution in methanol to obtain the crude product, dissolve the crude product in tetrahydrofuran, pass through 0.45μm The organic filter membrane was concentrated...
Embodiment 2
[0036]Example 2: Synthesis of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amine oxide)fluorene]co-2,5-pyridine}(PF6NO25Py)
[0037] The synthetic route is as follows:
[0038]
[0039] (1) The monomer 2,5-dibromopyridine is directly purchased from Bailingwei Company and used after recrystallization with methanol;
[0040] (2) Preparation of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amino)fluorene]co-2,5-pyridine} (PF6N25Py);
[0041] The monomer 2,7-bis(trimethylene borate)-9,9'-bis(N,N-diethylhexyl-6-amino)fluorene (728mg, 1mmol) prepared in Example 1 , monomer 2,5-dibromopyridine (237mg, 1mmol) and 10mg tetraphenylphosphorous palladium catalysts are dissolved in the mixed solvent of 10ml toluene and 5ml tetrahydrofuran, add the sodium carbonate aqueous solution of 4ml 2mol / L, under argon Under the protection of the reflux reaction for 48 hours, then cooled to room temperature, the reaction solution was precipitated in methanol to obtain a crude product, the crude product was diss...
Embodiment 3
[0044] Example 3: Synthesis of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amine oxide)fluorene]co-3,5-pyridine}(PF6NO35Py)
[0045] The synthetic route is as follows:
[0046]
[0047] (1) The monomer 3,5-dibromopyridine is directly purchased from Bailingwei Company and used after recrystallization with methanol;
[0048] (2) Preparation of poly{2,7-[9,9'-bis(N,N-diethylhexyl-6-amino)fluorene]co-3,5-pyridine} (PF6N35Py)
[0049] The monomer 2,7-bis(trimethylene borate)-9,9'-bis(N,N-diethylhexyl-6-amino)fluorene (728mg, 1mmol) prepared in Example 1 , monomer 3,5-dibromopyridine (237mg, 1mmol) and 10mg tetraphenylphosphorous palladium catalysts are dissolved in the mixed solvent of 10ml toluene and 5ml tetrahydrofuran, add the sodium carbonate aqueous solution of 4ml 2mol / L, under argon Under the protection of the reflux reaction for 48 hours, then cooled to room temperature, the reaction solution was precipitated in methanol to obtain a crude product, the crude product was diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com