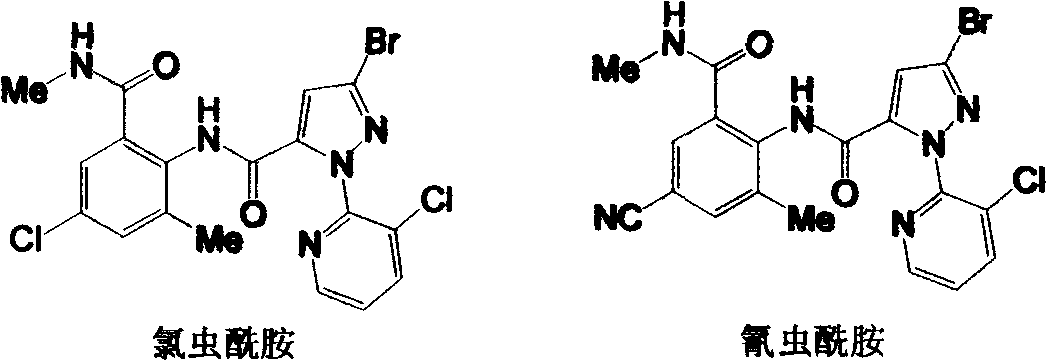
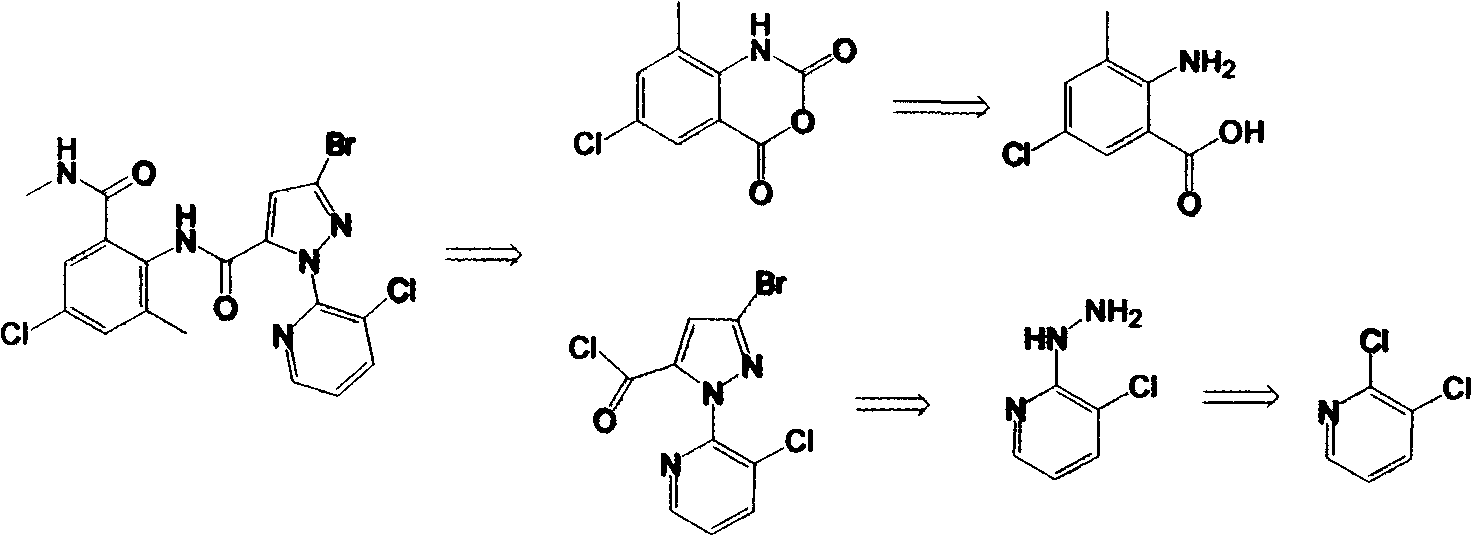
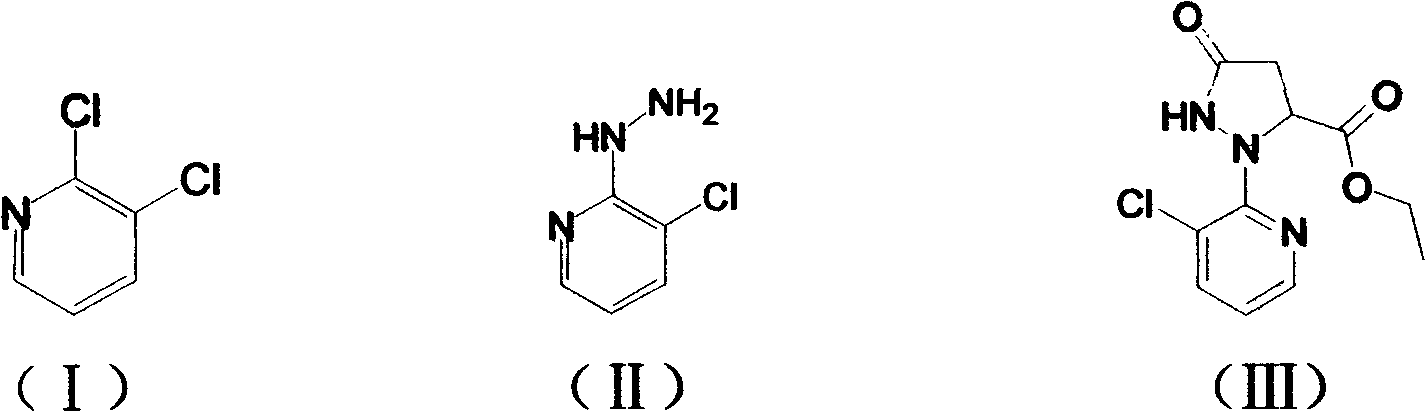
Preparation methods for important intermediates of anthranilic diamide compound
A technology of o-formamidobenzamide and compounds, which is applied in the field of chemical synthesis, can solve the problems of complex operation, low yield, long reaction time, etc., and achieve the effect of simple operation process, high product purity and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 2-hydrazino-3-chloropyridine (II)
[0033] Add 2,3-dichloropyridine (14.8g, 0.1mol) and 1,4-dioxane (120mL) into a reaction flask with a stirrer, and stir to dissolve at room temperature. After completely dissolving, pour into the reaction flask Slowly add 75ml (1.2mol) of 80% hydrazine hydrate dropwise, and drop it within 1 hour. At this time, the solution becomes clear, then the reaction system is heated to reflux state, reacted for 6 hours, and monitored with a TLC plate until the raw material 2,3- Dichloropyridine disappeared, stopped heating, cooled down to room temperature, and a light gray solid precipitated out. After suction filtration, washing with water and drying, 14.2 g of 2-hydrazino-3-chloropyridine solid was obtained, with a yield of 96% and a purity of 98%.
Embodiment 2
[0034] Embodiment 2: Preparation of 2-hydrazino-3-chloropyridine (II)
[0035] Add 2,3-dichloropyridine (14.8g, 0.1mol) and tetrahydrofuran (120mL) into a reaction flask with a stirrer, start stirring at room temperature until completely dissolved, then slowly add 80% hydrated Hydrazine 75ml (1.2mol), dripped in 1 hour, then the reaction system was heated to reflux state, reacted for 12 hours, monitored with TLC board until the raw material 2,3-dichloropyridine disappeared, stopped heating, cooled down to room temperature, A light gray solid was precipitated. After suction filtration, water washing and drying, 12.1 g of 2-hydrazino-3-chloropyridine solid was obtained, with a yield of 82% and a purity of 98%.
Embodiment 3
[0036] Embodiment 3: Preparation of 2-hydrazino-3-chloropyridine (II)
[0037] Add 2,3-dichloropyridine (14.8 g, 0.1 mol) and absolute ethanol (120 mL) into a reaction flask with a stirrer, and start stirring to dissolve at room temperature. After completely dissolving, slowly add 80% 75ml (1.2mol) of hydrazine hydrate was dropped within 1 hour, and the solution became clear at this time, then the reaction system was heated to reflux state, reacted for 6 hours, and monitored with a TLC plate until the disappearance of the raw material 2,3-dichloropyridine, Heating was stopped, cooled down to room temperature, and a light gray solid precipitated out. After suction filtration, washing with water and drying, 10.4 g of 2-hydrazino-3-chloropyridine solid was obtained, with a yield of 70% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com