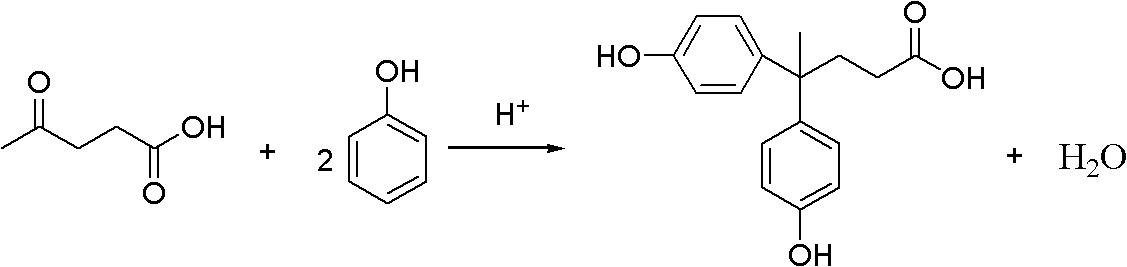
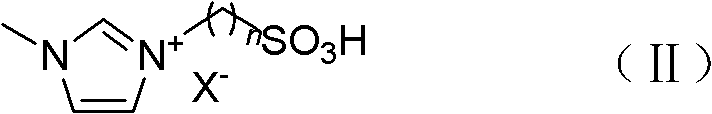Method for preparing diphenolic acid in ionic liquid
An ionic liquid and bisphenolic acid technology, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult recovery of catalysts, easy corrosion of equipment, and high production costs, and achieves low price and operation. Convenience and productivity-enhancing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1. Nucleophilic addition reaction
[0061] Add 208.3g (1.0mol) bisulfate 1-ethyl-3-methylimidazole ([Emim]HSO 4 ) and 94.1g (1.0mol) phenol, stirred and mixed; then in the reactor, 29.0g (0.25mol) levulinic acid and 1.9g (0.015mol) procatalyst sodium sulfite were added successively, stirred and mixed to obtain a mixture; Slowly heat the reactor, the reactor is heated to 40 ° C, and the mixture is subjected to a nucleophilic addition reaction under stirring to obtain a reaction mixture; wherein, the molar ratio between levulinic acid, phenol and the ionic liquid in the mixture is 1: 4: 4, the mol ratio of levulinic acid and cocatalyst is 1: 0.06, the temperature of control reaction is 40 ℃, and the reaction time is 20 hours;
[0062] 2. Separate the reaction mixture
[0063] Add 1.0L of cooled deionized water (4°C) to the above reaction mixture, stir and mix thoroughly for 5 minutes, then stand and separate layers to obtain an upper organic phase and a lower aqueous ph...
Embodiment 2
[0072] In addition to the nucleophilic addition reaction step, 368.4g (1.0mol) trifluoromethanesulfonate 1-methyl-3-butanesulfonate imidazole ionic liquid ([BSmim] CF 3 SO 3 ) and 94.1g (1.0mol) phenol, stirred and mixed; then in the reactor, 29.0g (0.25mol) levulinic acid and 1.9g (0.015mol) procatalyst sodium sulfite were added successively, stirred and mixed to obtain a mixture; Slowly heat the reactor, the reactor is heated to 60 ° C, and the mixture is subjected to a nucleophilic addition reaction under stirring to obtain a reaction mixture; wherein, the molar ratio between levulinic acid, phenol and the ionic liquid in the mixture is 1: 4: 4, the mol ratio of levulinic acid and cocatalyst is 1: 0.06, and the control reaction temperature is 60 ℃, and the reaction time is 20 hours, all the other are identical with embodiment 1, and the productive rate of bisphenolic acid is 92.5%;
[0073] bisphenolic acid 1 The HNMR nuclear magnetic characterization data is: 1 HNMR (DM...
Embodiment 3
[0075] In addition to the nucleophilic addition reaction step, add 316.3g (1.0mol) bisulfate radical 1-methyl-3-butanesulfonic acid imidazolium ionic liquid ([BSmim]HSO) successively in the reactor 4 ) and 141.2g (1.5mol) phenol, stir and mix; then add 29.0g (0.25mol) levulinic acid and 0.8g (0.005mol) co-catalyst sodium thiosulfate successively to the reactor, stir and mix to prepare Obtain the mixture; slowly heat the reactor, the reactor is heated to 50°C, and the mixture is subjected to a nucleophilic addition reaction under stirring to obtain a reaction mixture; wherein, the molar ratio between levulinic acid, phenol and the ionic liquid in the mixture It is 1: 6: 4, and the mol ratio of levulinic acid and procatalyst is 1: 0.02, and the control reaction temperature is 50 ℃, and the reaction times is 15 hours, and all the other are identical with embodiment 1, and the productive rate of bisphenolic acid is 83.2%;
[0076] bisphenolic acid 1 The HNMR nuclear magnetic chara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com