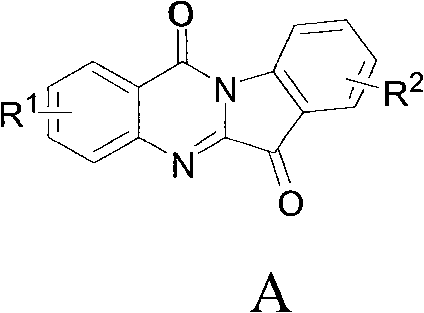
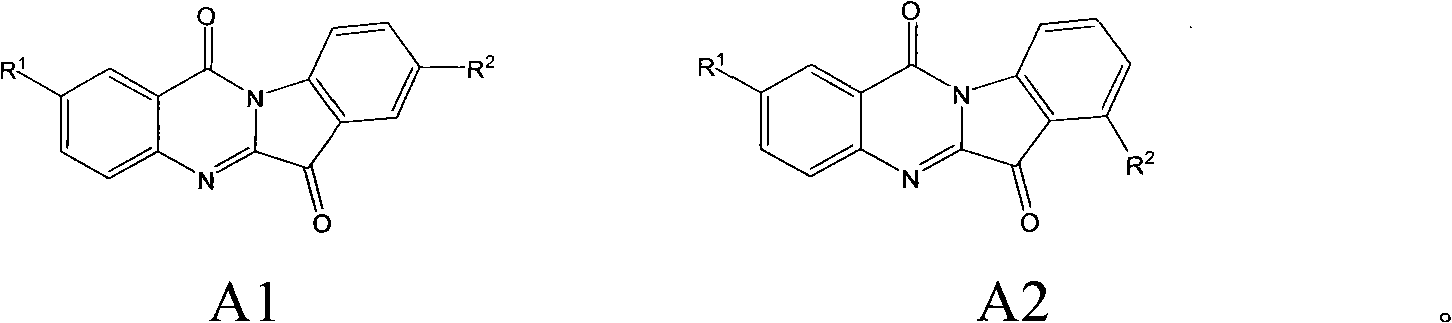
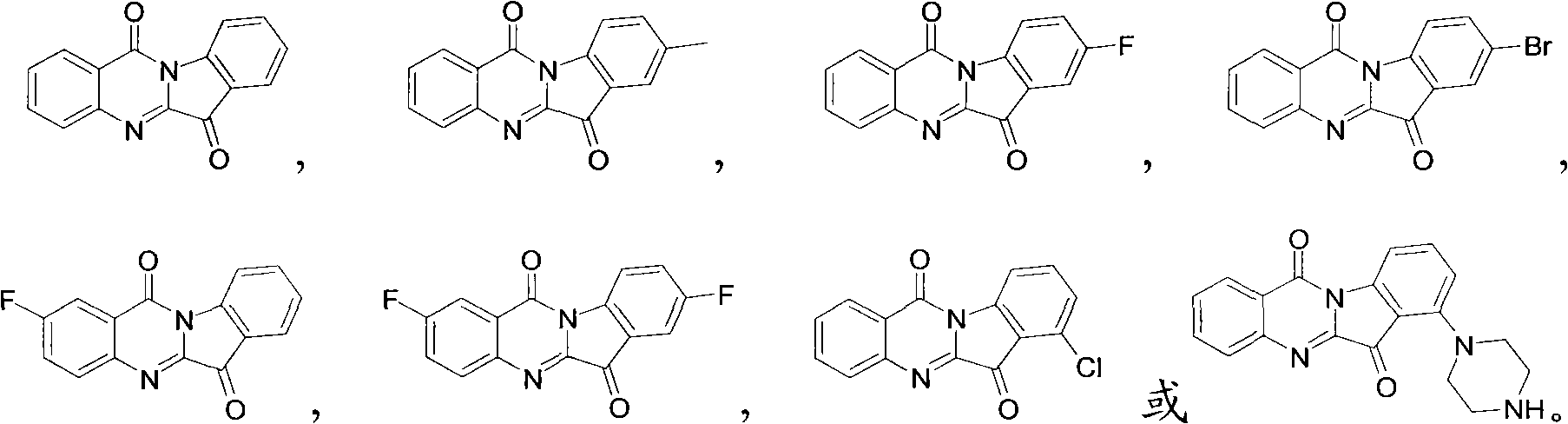
Preparation method of tryptanthrin compound and new application of tryptanthrin compound in preparing indoleamine-2,3-dioxygenase (IDO) inhibitor
A compound and inhibitor technology, applied in antiviral agents, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of low extraction rate, long separation process, difficult to meet research and clinical drug use, etc., to achieve good research with the development of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of tryptanthrin (formula I)
[0024]
[0025] Add 3mL toluene, 163mg (1mmol) isatoic anhydride (purchased from Shanghai Kangtuo Chemical Co., Ltd.), 147mg (1mmol) isatin (purchased from Aladdin Reagent Co., Ltd.), 505mg (5mmol) to a 25mL dry round bottom flask ) triethylamine, then heated up to 110°C, refluxed and stirred for 3-4h, after TLC detection showed that the reaction was complete, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethanol to obtain 228 mg of compound of formula I with a yield of 92%.
[0026] The characterization data are as follows: 1 H-NMR (400MHz, CDCl 3 )δ=8.64(d, 1H), 8.45(d, 1H), 8.04(d, 1H), 7.92(d, 1H), 7.88(t, 1H), 7.80(t, 1H), 7.69(t, 1H ), 7.44(t, 1H).
Embodiment 2
[0027] Embodiment 2: the preparation of 8-methyl tryptanthrin (formula II)
[0028]
[0029] Add 3 mL of toluene, 163 mg (1 mmol) isatoic anhydride, 161 mg (1 mmol) 5-methyl isatin (purchased from Qingdao Hexing Fine Chemical Co., Ltd.), 505 mg (5 mmol) triethylamine into a 25 mL dry round bottom flask , and then heated up to 110° C., refluxed and stirred for 3-4 hours. TLC showed that after the reaction was complete, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethanol to obtain 235 mg of the compound of formula II with a yield of 90%.
[0030] The characterization data are as follows: 1 H-NMR (400MHz, CDCl 3 )δ=8.49(d, 1H), 8.43(d, 1H), 8.02(d, 1H), 7.84(t, 1H), 7.67(m, 2H), 7.59(d, 1H), 2.46(s, 3H ).
Embodiment 3
[0031] Embodiment 3: the preparation of 8-fluorotryptanthin (formula III)
[0032]
[0033] Add 3 mL of toluene, 163 mg (1 mmol) isatoic anhydride, 165 mg (1 mmol) 5-fluoroisatin (see step (2) of Example 5 for its preparation method, 505 mg (5 mmol) three Ethylamine, then warmed up to 110°C, refluxed and stirred for 3-4 hours, TLC showed that after the reaction was completed, the solvent was evaporated under reduced pressure, and the residue was recrystallized from ethanol to obtain 242 mg of compound of formula III, with a yield of 91%.
[0034] The characterization data are as follows: 1 H-NMR (400MHz, CDCl 3 )δ=8.64(m, 1H), 8.44(d, 1H), 8.03(d, 1H), 7.87(t, 1H), 7.70(t, 1H), 7.58(m, 1H), 7.49(m, 1H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com