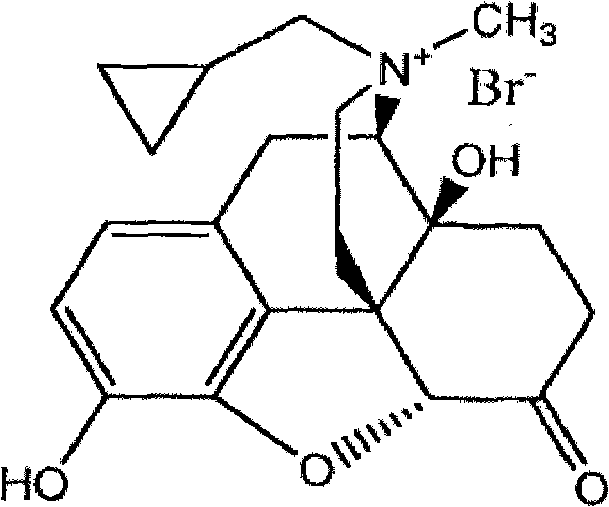
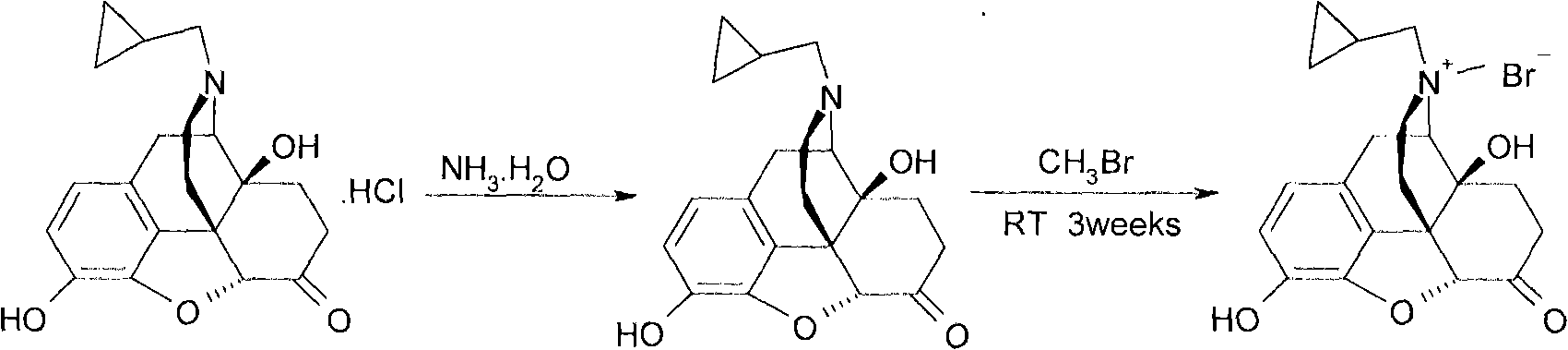
Preparation method of methyhaaltrexone bromide
A technology of methylnaltrexone bromide and naltrexone base, which is applied in the field of chemical drug synthesis, can solve the problems of high boiling point of dimethylformamide, impact on the quality of the final product, and excessive dimethylformamide, so as to reduce production costs , easy to refine, to ensure the effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
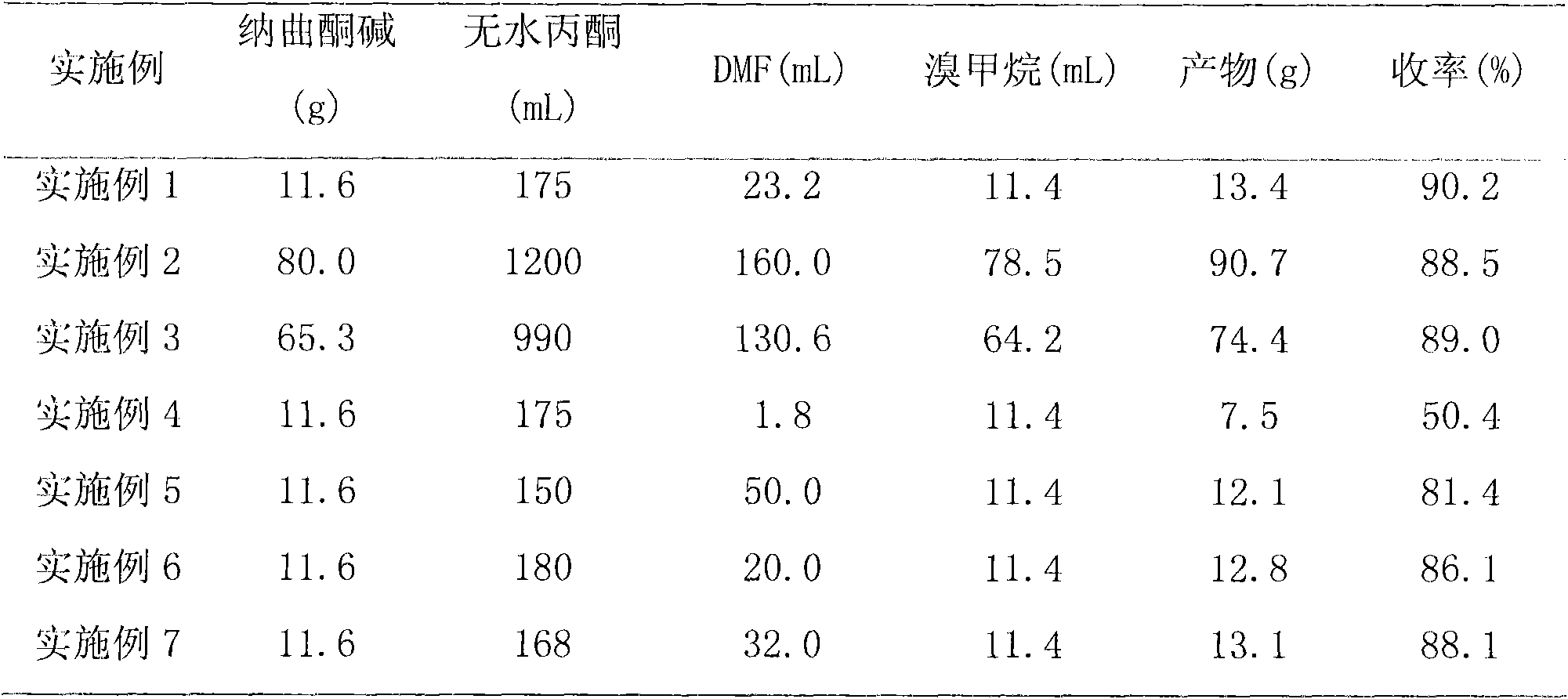
[0028] Under nitrogen protection, add 11.6g (34.0mmol) naltrexone base, 175mL anhydrous acetone, 23.2mL (23.2mL DMF), 11.4mL (208mmol) methyl bromide to the three-necked flask, turn off the nitrogen, and react in a closed atmosphere at 25°C for 21 days. After the reaction is completed, reduce the pressure. The reaction solution was concentrated to dryness, washed with 150 mL of acetone, and the filter cake was collected by suction filtration, and vacuum-dried at 80° C. for 6 h to obtain 13.4 g of bromonaltrexone product with a yield of 90.17%.
Embodiment 2
[0030] Under nitrogen protection, add 80.0 g of naltrexone base, 1200 mL of anhydrous acetone, 160.0 mL of DMF, 78.5 mL of methyl bromide to the three-necked flask, turn off the nitrogen, and seal the reaction at 25°C for 21 days. After the reaction is completed, the reaction solution is concentrated under reduced pressure to dryness, and added After washing with 1000 mL of acetone, the filter cake was collected by suction filtration, and vacuum-dried at 80 °C for 6 h to obtain 90.7 g of methylnaltrexone product with a yield of 88.5%.
Embodiment 3
[0032] 65.3g of naltrexone base, 990mL of anhydrous acetone, 130.6mL of DMF, 64.2mL of methyl bromide were added to the three-necked flask, and the reaction was airtight at 25°C for 21 days. After the reaction was completed, the reaction solution was concentrated under reduced pressure to dryness, and 800mL of acetone was added for washing and suction filtration. The filter cake was dried under vacuum at 80°C for 6 hours to obtain 74.4 g of methylnaltrexone bromide product with a yield of 89.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com