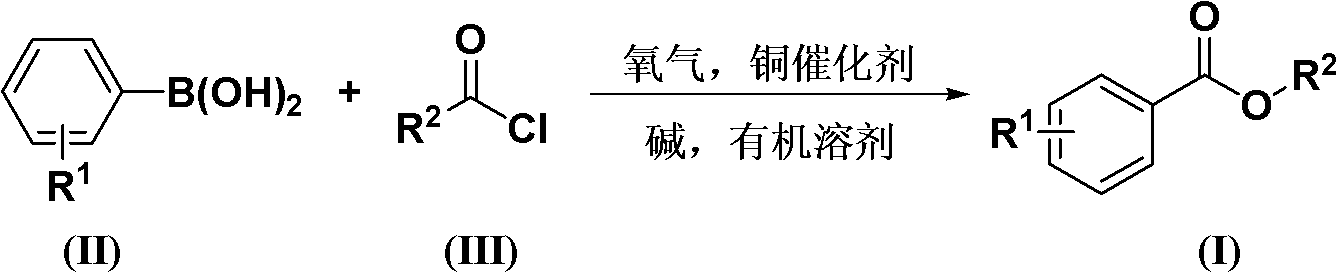
Method for synthesizing aromatic formic acid aryl ester derivative
A synthetic method, the technology of aryl boronic acid, applied in the preparation of carboxylic acid halides, organic chemistry, etc., can solve the problems of poor functional group compatibility, reduced yield, low reaction selectivity, etc., and achieve great implementation value and social Economic benefit, good product quality, reaction selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] According to the molar ratio of acid chloride, aryl boronic acid, copper catalyst, ligand, basic compound is 1.0: 1.5: 0.1: 0.2: 2.0 feeding intake; acid chloride is benzoyl chloride, feeding quality 14.1g (0.1mol); aryl boronic acid is Phenylboronic acid, feeding quality 18.3g (0.15mol); copper catalyst is copper acetate, feeding quality 1.82g (0.01mol); ligand is 1,4-diazabicyclo[2.2.2]octane (DABCO), The feeding quality is 2.24g (0.02mol); the basic compound is potassium carbonate, and the feeding quality is 27.6g (0.2mol); the organic solvent is toluene 169g, and its total consumption is 12 times of the quality of benzoyl chloride.
[0032] In an oxygen atmosphere, put benzoyl chloride, phenylboronic acid, copper acetate, 1,4-diazabicyclo[2.2.2]octane (DABCO), and potassium carbonate into the reaction kettle at room temperature, add toluene to dissolve, The reaction temperature was 110°C, and the reaction was completed after 24 hours.
[0033] After the reaction wa...
Embodiment 2
[0036] According to the molar ratio of acid chloride, aryl boronic acid, copper catalyst, ligand, basic compound is 1.0: 1.5: 0.1: 0.2: 2.0 feeding intake; acid chloride is benzoyl chloride, feeding quality 14.1g (0.1mol); aryl boronic acid is Phenylboronic acid, feeding quality 18.3g (0.15mol); copper catalyst is copper acetylacetonate, feeding quality 2.62g (0.01mol); ligand is 1,4-diazabicyclo[2.2.2]octane (DABCO) , feed quality 2.24g (0.02mol); Basic compound is potassium carbonate, feed quality 27.6g (0.2mol); Organic solvent is toluene 169g, and its total consumption is 12 times of benzoyl chloride quality.
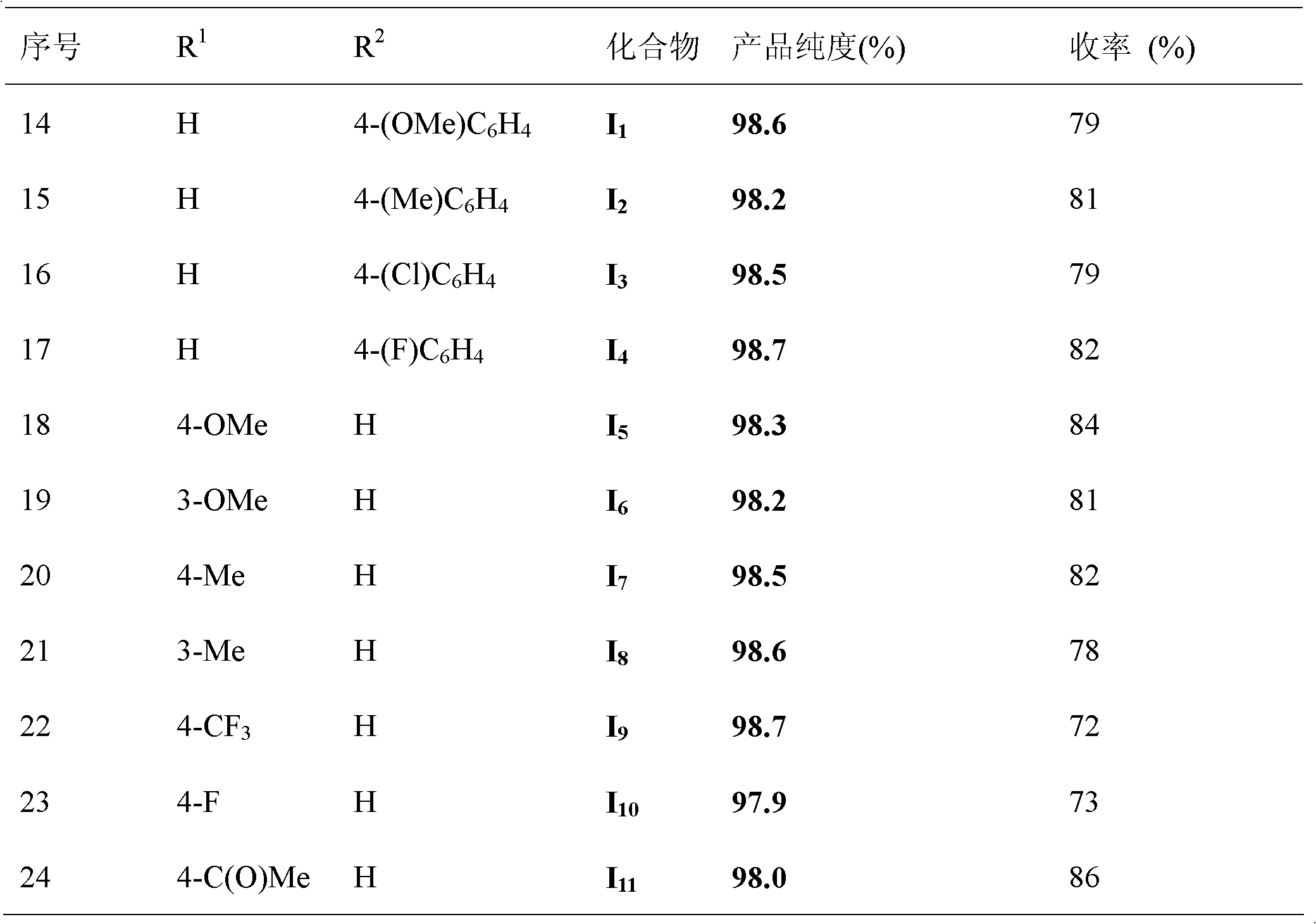
[0037] All the other are with embodiment 1, gained product phenyl benzoate 14.5g, yield 73%, purity 98.5%.
Embodiment 3
[0039]According to the molar ratio of acid chloride, aryl boronic acid, copper catalyst, ligand, basic compound is 1.0: 1.5: 0.1: 0.2: 2.0 feeding intake; acid chloride is benzoyl chloride, feeding quality 14.1g (0.1mol); aryl boronic acid is Phenylboronic acid, feeding quality 18.3g (0.15mol); copper catalyst is cuprous acetate, feeding quality 1.23g (0.01mol); ligand is 1,4-diazabicyclo[2.2.2]octane (DABCO) , feed quality 2.24g (0.02mol); Basic compound is potassium carbonate, feed quality 27.6g (0.2mol); Organic solvent is toluene 169g, and its total consumption is 12 times of benzoyl chloride quality.
[0040] All the other are with embodiment 1, gained product phenylbenzoate 12.5g, yield 63%, purity 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com