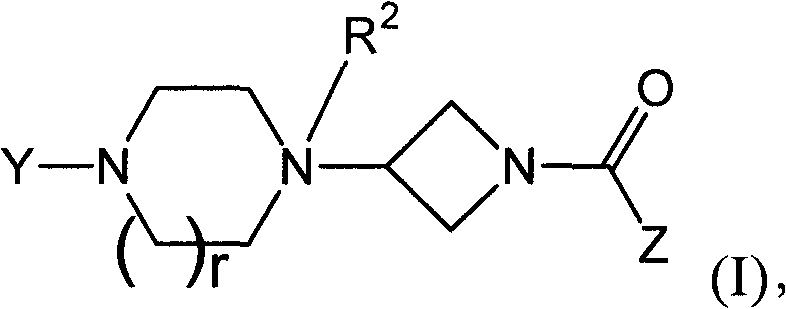
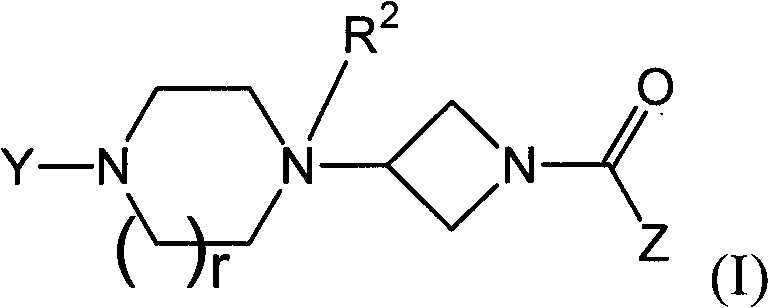
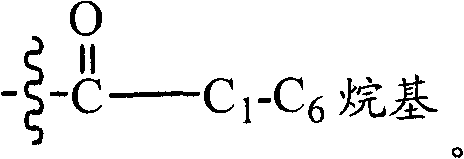Heteroaromatic and aromatic piperazinyl azetidinyl amides as monoacylglycerol lipase inhibitor
A heteroaryl and triazine-based technology, applied in the field of novel monoacylglycerol lipase inhibitors, can solve the problem that it is difficult to separate beneficial effects from harmful side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0456] Example 1: Compound #12
[0457] Biphenyl-4-yl-[3-(4-pyrimidin-2-yl-piperazin-1-yl)-azetidin-1-yl]-methanone
[0458]
[0459] Step A: 2-[4-(1-Benzhydryl-azetidin-3-yl)-piperazin-1-yl]-pyrimidine
[0460] 2-Piperazin-1-yl-pyrimidine (2.48g, 15.10mmol, Alfa) and 1-benzhydryl-azetidin-3-yl methanesulfonate (4g, 12.6mmol, Oakwood) CH 3 To the solution of CN (40 mL) was added DIPEA (2.63 mL, 15.10 mmol). The resulting mixture was then refluxed for 2 h. The solvent was removed by evaporation and the residue was dissolved in CH 2 Cl 2 and NaHCO 3 Partition between aqueous solutions. with NaHCO 3 The organic layer was washed with aqueous solution (2x), then extracted with 1N HCl (2x). The aqueous layer was cooled, then the pH was adjusted with 1N NaOH. The resulting mixture was treated with CH 2 Cl 2 (2x) extraction. The organic layer was washed with MgSO 4 Dry and concentrate. The resulting residue was purified by MPLC to afford 2-[4-(1-benzhydryl-azet...
example 2
[0480] Example 2: Compound #139
[0481] (6-Trifluoromethyl-benzo[b]thiophen-2-yl)-[3-(4-pyrimidin-2-yl-piperazin-1-yl)-aza Cyclobutan-1-yl]-methanone
[0482]
[0483] Step A: tert-butyl 4-(2,2,2-trifluoro-acetyl)-piperazine-1-carboxylate
[0484] CH 2 Cl 2 (100mL) solution was added dropwise (CF 3 CO) 2 O (10.5 mL, 75.54 mmol). The resulting mixture was stirred at 0 °C for 2 h. Then 2N HCl (60 mL) was added. The organic layer was washed with MgSO 4 Dry, filter and concentrate. The resulting residue (title compound) was used in the next reaction without further purification. MS m / z (MH + -Boc)183.1, (MH + -C 4 h 9 )227.1; 1 H NMR (300MHz, CDCl 3 ): δ3.45-3.7 (m, 8H), 1.5 (s, 9H).
[0485] Step B: 2,2,2-Trifluoro-1-piperazin-1-yl-ethanone
[0486] CH 2 Cl 2 (60 mL) was added trifluoroacetic acid (18 mL). The resulting mixture was stirred at room temperature for 18 h. Solvent was removed by evaporation. Diethyl ether (100 mL) was added to the ...
example 3
[0500] Example 3 (in vitro analysis): MGL enzyme activity assay
[0501] All rate-based assays were performed in black 384-well polypropylene PCR microplates (Abgene) in a total volume of 30 μL. The substrate butyrate-4-methylumbelliferone (4MU-B; Sigma) and purified mutant MGL enzyme (mut-MGLL 11-313 L179S L186S) or purified wild-type MGL (wt-MGLL 6H-11- 313) were respectively diluted into 20 mM PIPES buffer (pH=7.0) containing 150 mM NaCl and 0.001% Tween 20. Compounds of formula (I) were pre-dispensed (50 nL) into assay plates using a Cartesian Hummingbird (Genomic Solutions, Ann Arbor, MI), followed by the addition of 4MU-B (25 μL of a 1.2X solution to a final concentration of 10 μM), followed by enzyme ( 5 μL of 6X solution to a final concentration of 5 nM) to initiate the reaction. Final compound concentrations ranged from 17 to 0.0003 [mu]M. Excitation and emission wavelengths of 335nm and 440nm at 37°C, respectively, and a bandwidth of 10nm (Safire 2 , Tecan) to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com