Method for improving transient expression of recombinant proteins in mammalian cells through temperature jump
A temperature mutation, mammalian technology, applied in the biological field, can solve the problem of not enhancing the transient transfection efficiency of cells, achieve great commercial value, and improve the effect of transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
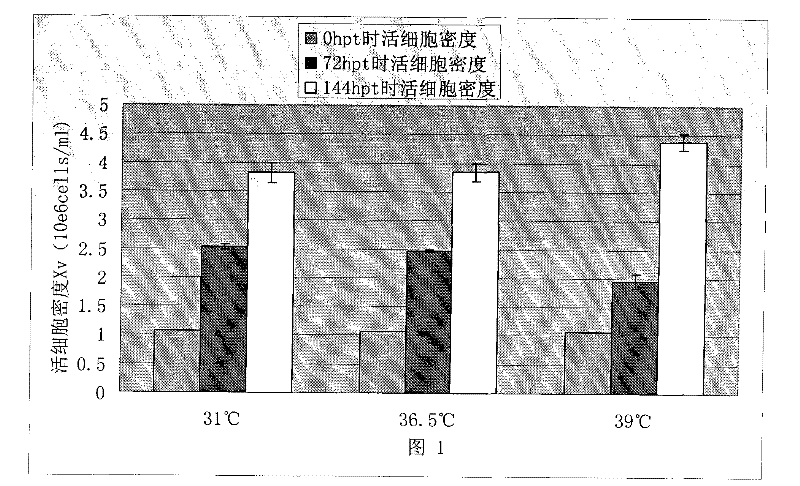
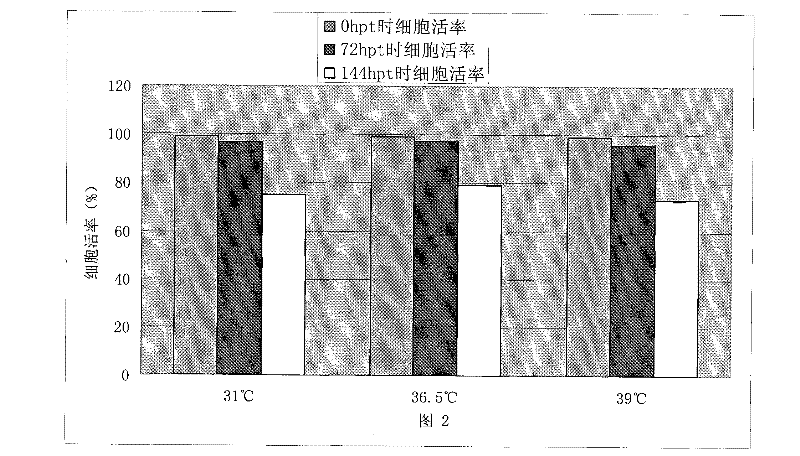
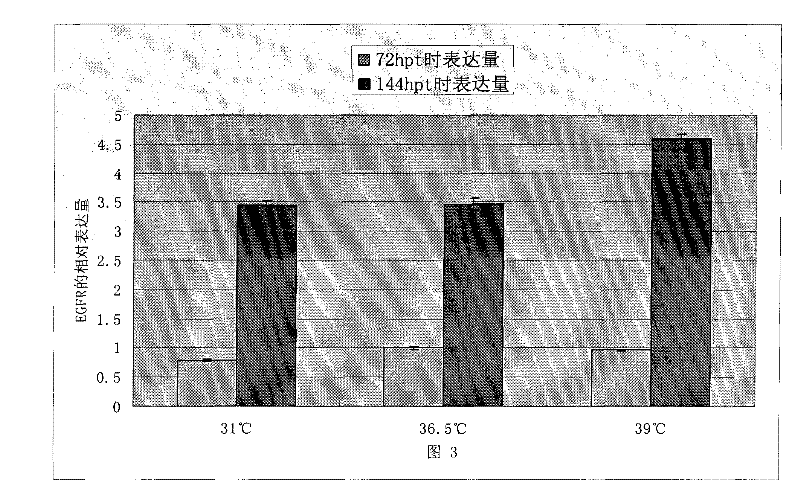
[0051] Embodiment 1: The influence of temperature mutation on HEK 293 cell recombinant EGFR protein expression
[0052] In order to analyze the impact of the mutation of the temperature of the cell environment during transfection on the transient transfection process of the cells, we used the plasmid encoding the EGFR protein with the Fc tag to perform the following experiments. One day before transfection, a total of 9 flasks of HEK 293 cells were inoculated and cultured in a shaker at 36.5°C. Three hours before transfection, 3 bottles were taken respectively and placed in a shaker at a temperature of 31°C and 39°C, and the other 3 bottles were continued to be placed in a shaker at 36.5°C. Transfection was performed 3 hours later. After adding the exogenous DNA-transfection reagent complex, the shake flasks were put back into the shakers at 31°C, 36.5°C and 39°C respectively. After 3 hours, all shake flasks were placed in a shaker at 36.5°C for incubation. All cell DNA-tra...
Embodiment 2
[0054] Example 2: Determination of the start time of temperature mutation in HEK 293 cells
[0055] The following experiment was designed to determine the start time of temperature mutation of HEK 293 cells: one day before transfection, a total of 18 flasks of HEK 293 cells were inoculated, divided into 6 groups, with 3 parallel samples in each group, and cultured in a shaker at 36.5°C. On the next day, 4 hours, 3 hours, 2 hours, and 1 hour before transfection, a group of three shake flasks were taken out from the shaker at 36.5°C and placed in a shaker at 39°C. These 4 groups of cells continued to be placed in a shaker at 39°C for 3 hours after the DNA-transfection reagent complex was added to the cell culture medium, and then transferred to a shaker at 36.5°C to continue culturing. The cells in group 5 (ie, 0 hour group) were placed in a shaker at 36.5°C before transfection, and the DNA-transfection reagent complex was added to the cell culture medium and placed in a shaker ...
Embodiment 3
[0057] Example 3: Determination of duration of temperature mutation in HEK 293 cells
[0058]The following experiment was designed to determine the duration of the temperature mutation of HEK 293 cells: one day before transfection, a total of 18 flasks of normally treated cells were divided into 6 groups, with 3 parallel samples in each group, and they were cultured normally in a shaker at 36.5°C. Two hours before the staining, the five groups of cells were taken out from the shaker at 36.5°C and placed in a shaker at 39°C for culture. After 2 hours of transfection, after the DNA-transfection reagent complex was added to the cell culture medium, one group was taken and returned to a shaker at 36.5°C to continue culturing (ie, the 0-hour group). The other 4 groups were cultured in the shaker at 39°C, and one group was taken back to the shaker at 36.5°C at 1 hour, 2 hours, 3 hours and 4 hours after the DNA-transfection reagent complex was added to the cell culture medium. Conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com