Electrochemical stripping voltammetry for continuously measuring arsenic, stibonium and lead in mine groundwater
A stripping voltammetry and electrochemical technology, applied in the direction of material electrochemical variables, etc., to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Pretreatment of gold electrodes
[0032] With 0.05mm Al 2 o 3 The polishing powder mechanically grinds the gold electrode on the polishing paper for about two minutes, and thoroughly washes the gold electrode with secondary water after polishing; then electrochemically oxidizes and reduces the gold, and polarizes the electrode at 2V and -0.35V for 10s by chronoamperometry, respectively. Then at 0.5 M H 2 SO 4 Scan the active electrode for 10 circles in a medium cycle (scanning range: -300~1600mV, scanning rate: 4V / s); finally, at 0.5 M H 2 SO 4 The treated gold electrode was characterized by cyclic voltammetry (scan range: -300~1600mV, scan rate: 0.1V / s), the counter electrode used in the experiment was a platinum electrode, and the reference electrode was a saturated calomel electrode;
[0033] 2) Continuous determination of the concentration of arsenic, lead and antimony:
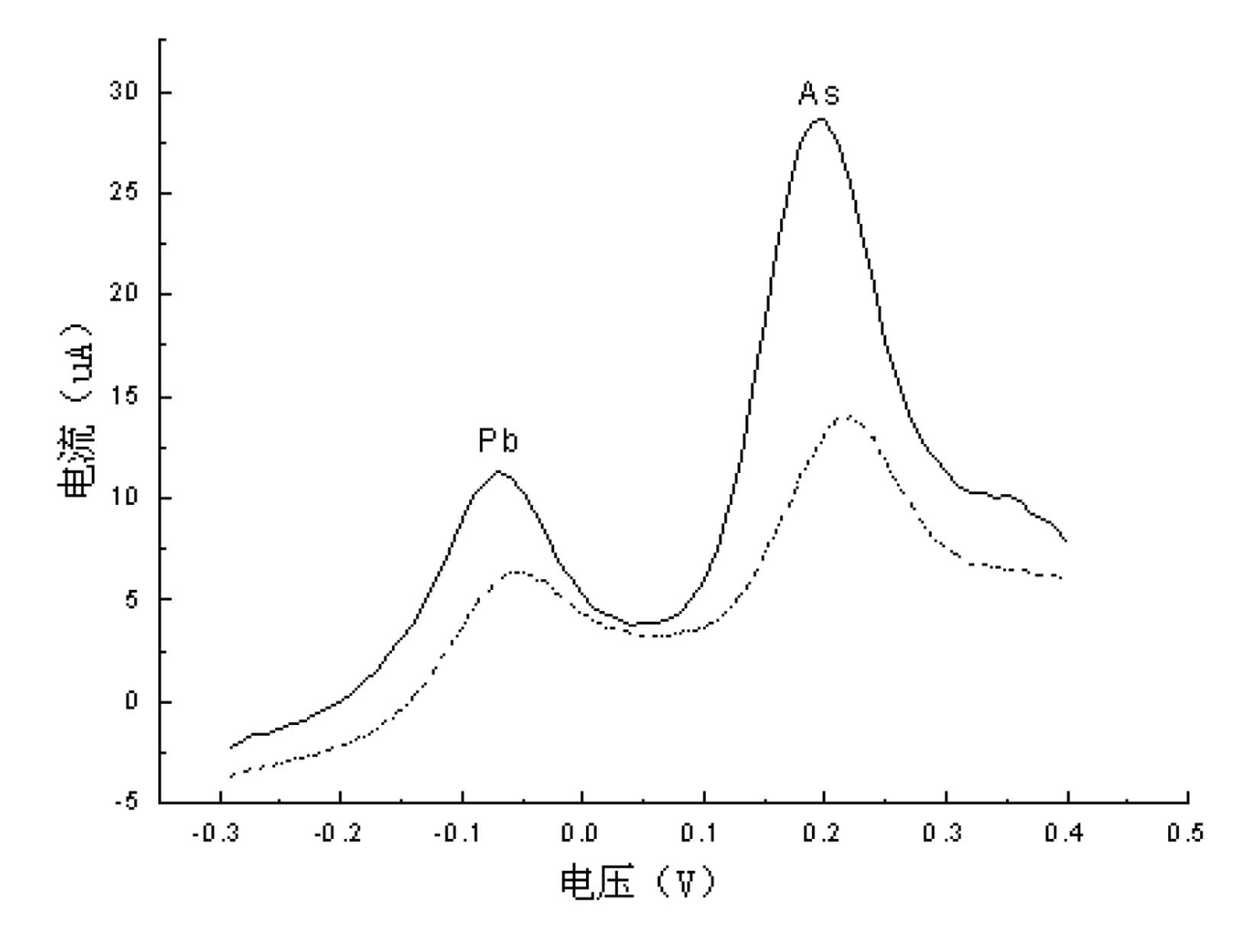
[0034] a) Continuously measure the concentration of As(Ⅲ), Pb(Ⅱ) solution
[0035] Us...
Embodiment 2
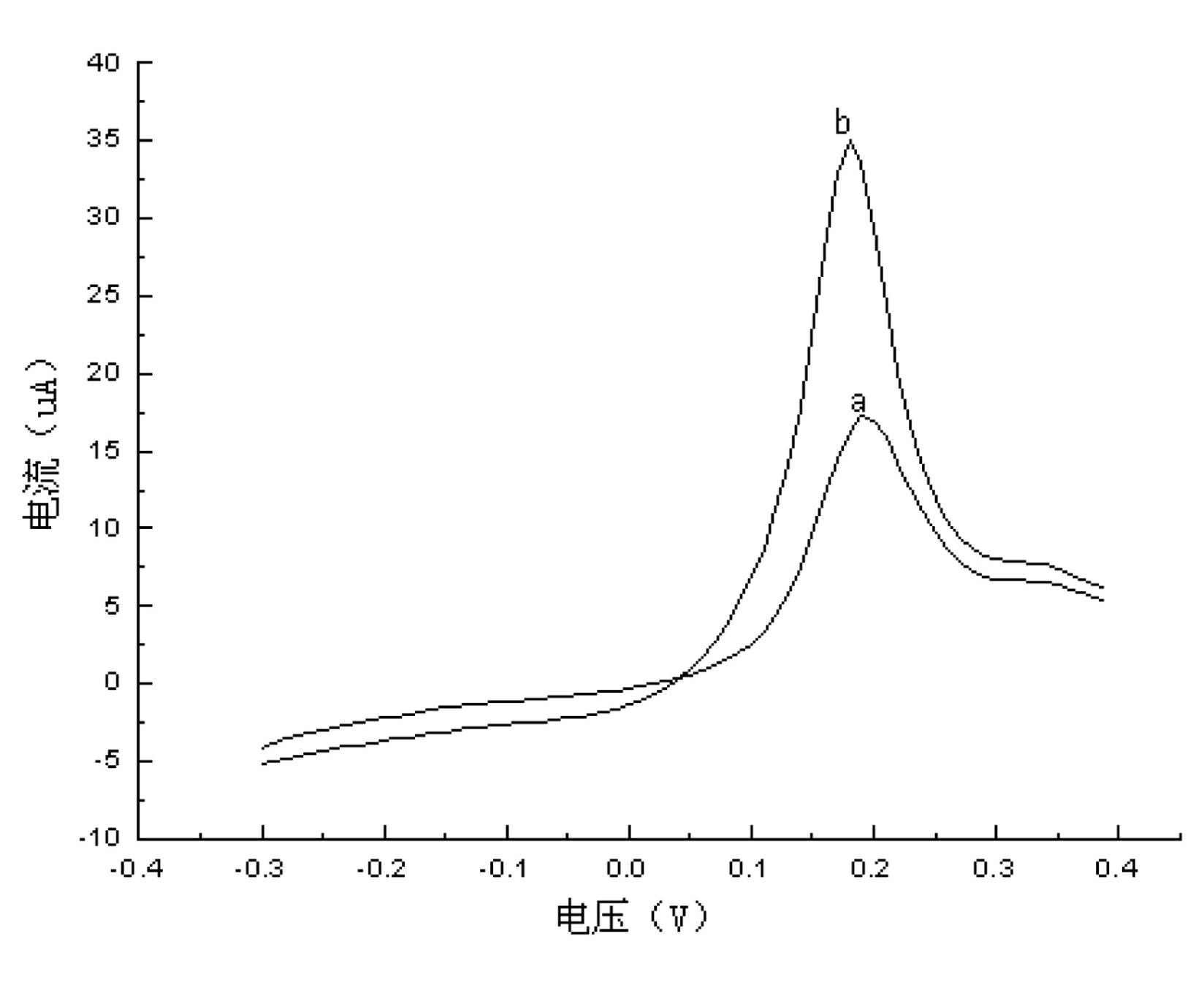
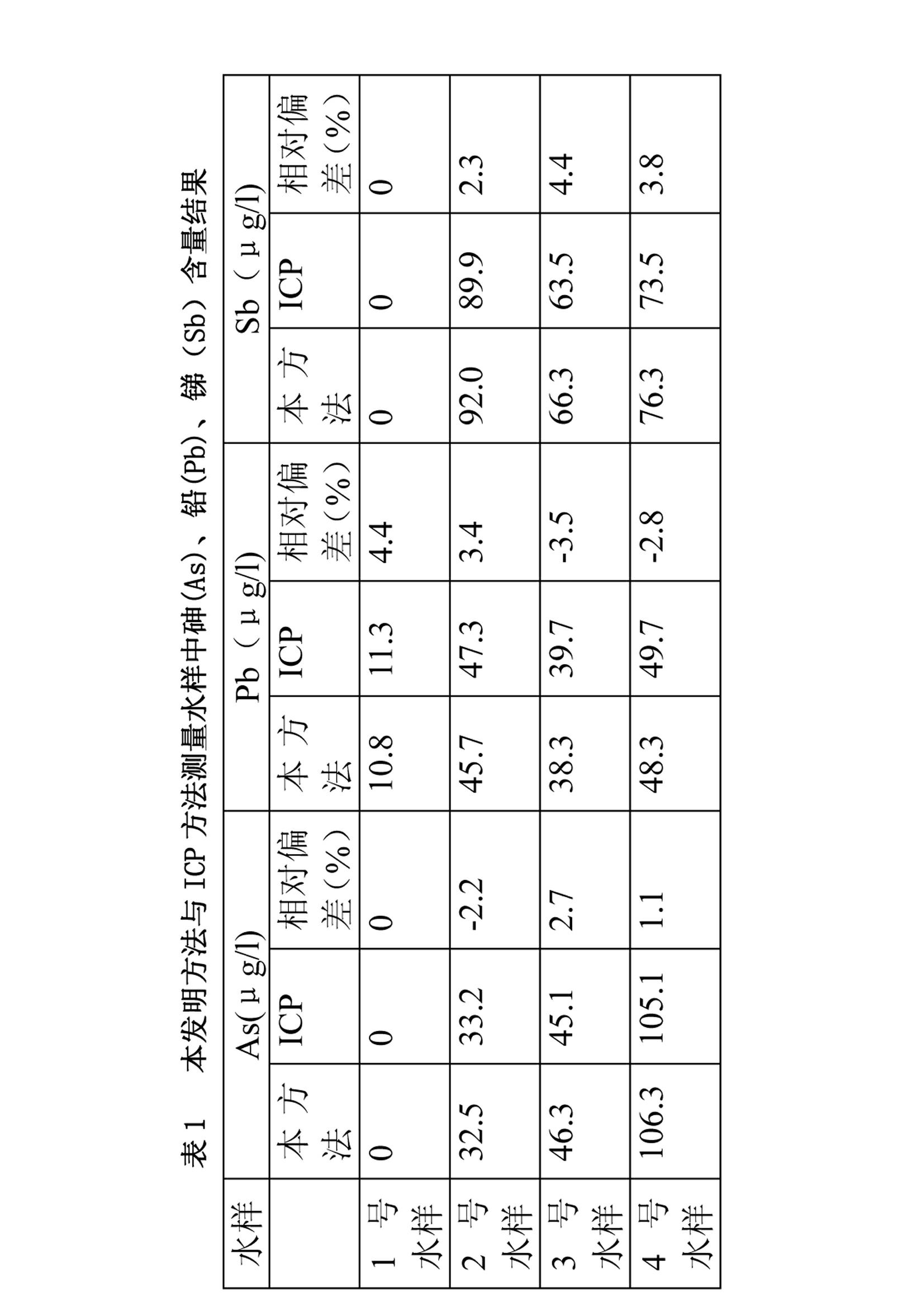
[0051] Determination of Arsenic, Lead and Antimony in Groundwater Samples of Mine
[0052] Take 50ml of mine groundwater sample and add H 2 SO 4 , HCl and Na 2 S0 3 , H in solution 2 SO 4 Concentration is 1M, HCl concentration is 0.1M, Na 2 S0 3 The concentration is 1 mg / l. Heat to a slight boil for 10 minutes and cool to room temperature.
[0053] Continuous determination of concentration of As(Ⅲ), Pb(Ⅱ) solution
[0054] During the determination process, the treated gold electrode was selected as the working electrode, the saturated calomel electrode was used as the reference electrode, and the platinum electrode was used as the counter electrode. The measurement conditions are as follows:
[0055] Electrodeposition potential -0.3V, electrodeposition time 180s (stirring 180s);
[0056] Rest potential -0.3V, rest time 30s;
[0057] Scan rate 100mV / s, scan range -0.3V-0.4V;
[0058] The measurement conditions for the continuous determination of the concentrations ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com