Reactive dark blue bisazo dye and preparation method thereof
A disazo dye, dark blue technology, used in reactive dyes, azo dyes, dyeing methods, etc., can solve the problems of insufficient lifting force and levelness, poor washing fastness, low color fixing rate, etc. The effect of deep dyeing ability, low soaping loss rate and good binding stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The active dark blue disazo dye of the present invention is prepared with reference to the existing conventional technology in this field, and the preparation method and process are simple to operate, and specifically include the following steps:
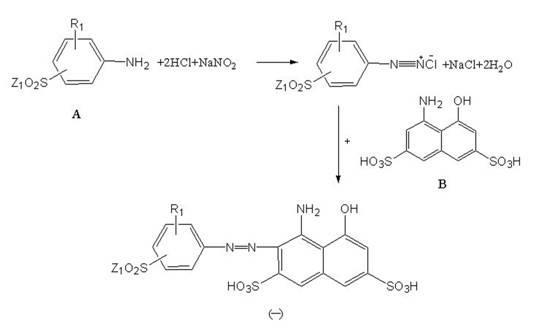
[0023] (1) Compound A is diazotized in the presence of hydrochloric acid and sodium nitrite, and the obtained diazotized product is acid-coupled with compound B to obtain compound (1); the reaction route is shown in formula II:
[0024]
[0025] II
[0026] (2) Compound C and compound D undergo dehydrohalogenation condensation reaction, and the obtained condensation product is further subjected to dehydrohalogenation condensation reaction with compound E to obtain compound (2); the reaction route is shown in formula III:
[0027]
[0028] III
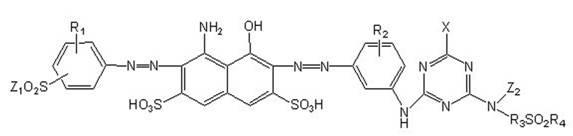
[0029] (3) After diazotization of the amino group of compound (2) obtained in step (2) and acidic coupling with compound (1) obtained in step (1), the reactive dark blue disazo ...
Embodiment 1
[0034] (1a), suspend 28.1g 4-(β-sulfatoethylsulfonyl)aniline in 200g water, wash with saturated NaHCO 3 (aq) Adjust the solution to neutral, dissolve 4-(β-sulfatoethylsulfonyl)aniline in water, then cool the solution to 0°C; add 7g of sodium nitrite, 50g of water to the solution And 30g concentrated hydrochloric acid, carry out diazotization reaction 2 hours. Then, use sulfamic acid to consume excess nitrite.
[0035] (1b), add dropwise 31.9g of 1-amino-8-hydroxynaphthalene-3,6 disulfonic acid to the solution after step (1a) (this acid is preferably pre-dissolved in 300g of water, and neutralized to neutral with alkali) , carry out the coupling reaction at a temperature of 5°C-15°C for 4-8 hours, and the structural formula of the obtained product is as follows:
[0036]
[0037] (2a) Dissolve 30.9g of N-2-(2-ethylsulfone sulfate)ethylaniline in 100g of water, adjust the pH to neutral, add dropwise 19.5g of N-2-(2-ethylsulfone sulfate) ethylaniline dissolved in 80g of a...
Embodiment 2
[0046] In this example, a reactive dark blue disazo dye (Ib) with the following structural formula was obtained. The preparation method was the same as in Example 1, except that the same molar equivalent (i.e. 15.5g) of cyanuric fluoride was used instead of cyanuric fluoride in step (2a). Uric acid chloride.
[0047]
[0048] The reactive dark blue disazo dye (Ib) prepared in this example is also a dark blue powder with a maximum absorption wavelength λmax=610nm in water, showing a dark blue color.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com