Cefmenoxime hydrochloride composition for injection and preparation thereof
A technology of cefmenoxime hydrochloride and a composition is applied in the field of cefmenoxime hydrochloride composition for injection, which can solve the problems of drug powder fluidity, poor stability, potential safety hazards, complicated process steps, etc., and achieves fast dissolution speed and increased collision. Opportunity, small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
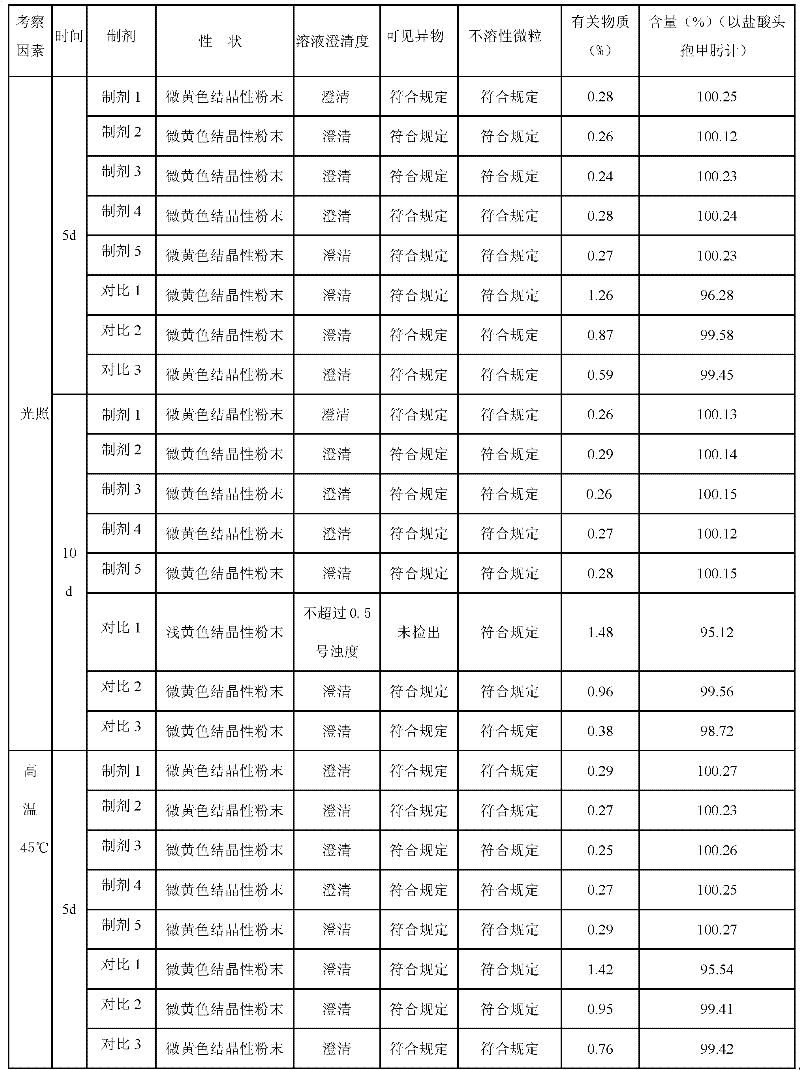
Image
Examples
Embodiment 1
[0043] The preparation method of embodiment 1 cefmenoxime hydrochloride composition
[0044] The composition comprises: 10 parts by weight of cefmenoxime hydrochloride crystals prepared by the invention, and 1.75 parts by weight of anhydrous sodium carbonate.
[0045] The preparation method is:
[0046] (1) take cefmenoxime hydrochloride crystal and anhydrous sodium carbonate in proportion, fully mix;
[0047] (2) sub-package into sterilized vials and stopper;
[0048] Wherein, the preparation method of cefmenoxime hydrochloride crystal is:
[0049] a. Take 0.5Kg of cefmenoxime hydrochloride solid and add 10L of water and stir to make a suspension, add 90g of anhydrous sodium carbonate at 15°C, stir and dissolve to obtain a clear liquid;
[0050] b. Adding the clarified liquid to 0.01% active carbon for decolorization by mass percentage, stirring the filtrate after 0.5 hours;
[0051] c. Add 2mol / L hydrochloric acid and an organic solvent to the filtrate obtained in step c...
Embodiment 2
[0053] The preparation method of embodiment 2 cefmenoxime hydrochloride composition
[0054] The composition comprises: 10 parts by weight of cefmenoxime hydrochloride crystals prepared by the invention, and 1.75 parts by weight of anhydrous sodium carbonate.
[0055] The preparation method is:
[0056] (1) take cefmenoxime hydrochloride crystal and anhydrous sodium carbonate in proportion, fully mix;
[0057] (2) sub-package into sterilized vials and stopper;
[0058] Wherein, the preparation method of cefmenoxime hydrochloride crystal is:
[0059] a. Take 0.5Kg of cefmenoxime hydrochloride solid and add 10L of water and stir to make a suspension, add 90g of anhydrous sodium carbonate at 15°C, stir and dissolve to obtain a clear liquid;
[0060] b. Adding the clarified liquid to 0.02% active carbon for decolorization by mass percent, and stirring the filtrate for 1 hour;
[0061] c. Add 4mol / L hydrochloric acid and an organic solvent to the filtrate obtained in step b at ...
Embodiment 3
[0063] The preparation method of embodiment 3 cefmenoxime hydrochloride composition
[0064] The composition comprises: 10 parts by weight of cefmenoxime hydrochloride crystals prepared by the invention, and 1.75 parts by weight of anhydrous sodium carbonate.
[0065] The preparation method is:
[0066] (1) take cefmenoxime hydrochloride crystal and anhydrous sodium carbonate in proportion, fully mix;
[0067] (2) sub-package into sterilized vials and stopper;
[0068] Wherein, the preparation method of cefmenoxime hydrochloride crystal is:
[0069] a. Take 0.5Kg of cefmenoxime hydrochloride solid and add 10L of water and stir to make a suspension, add 90g of sodium carbonate at 15°C, stir and dissolve to obtain a clear liquid;
[0070] b. Adding the clarified liquid to 0.05% activated carbon for decolorization by mass percent, stirring the filtrate after 1.5 hours;
[0071] c. Add 2mol / L hydrochloric acid and an organic solvent to the filtrate obtained in step b at 10°C u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Distribution width | aaaaa | aaaaa |
| Distribution width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com