Sodium hyaluronate injection sterilization method for guaranteeing aseptic packaging
A technology of sodium hyaluronate and sterilization methods, applied in heating and other directions, can solve the problems of destroying the airtightness of aseptic packaging, burst bottles, drug contamination and long bacteria, and achieve the effect of avoiding syringe bursting and less degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
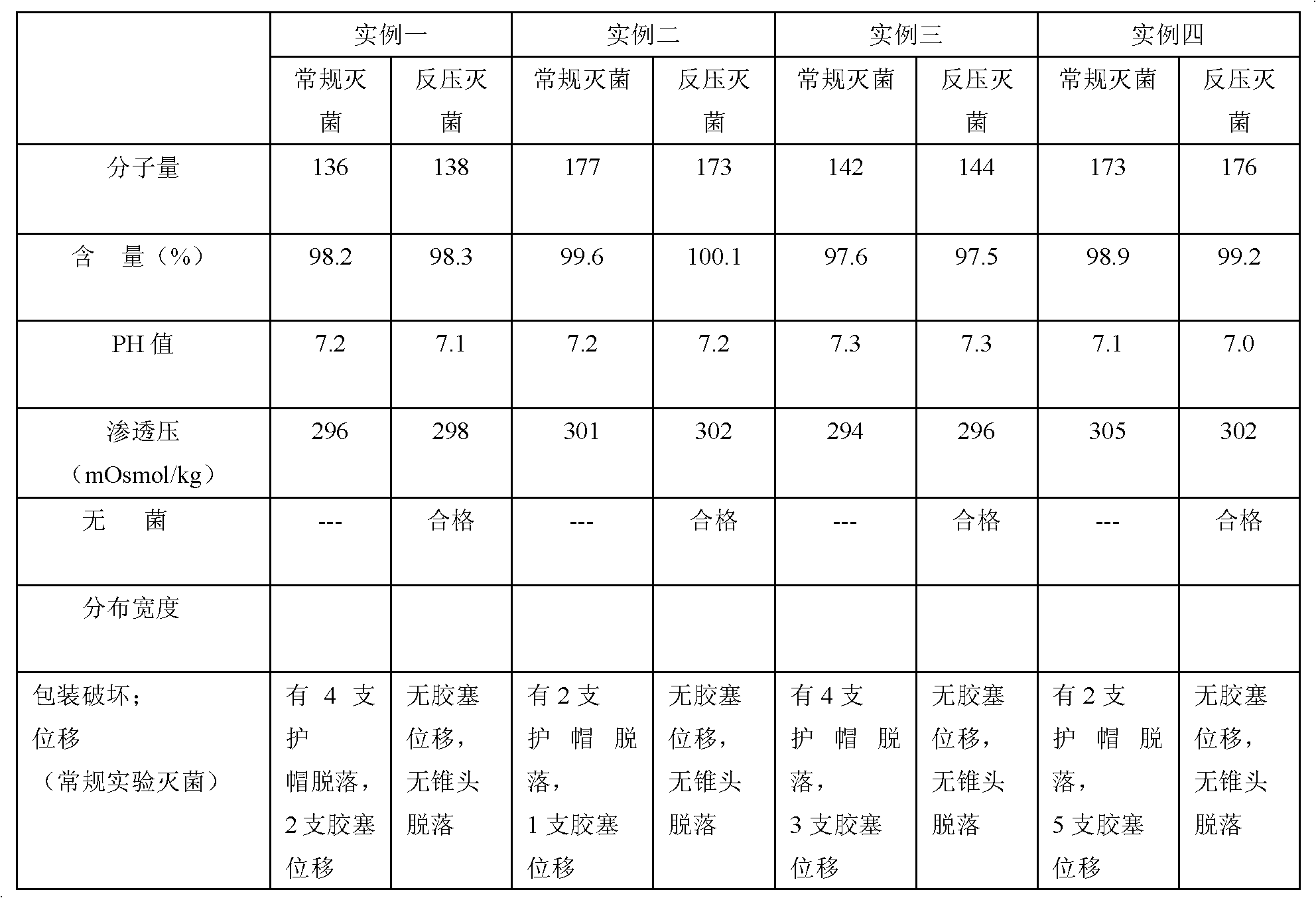
[0018] Take 200 bottles of 2.5ml:25mg sodium hyaluronate injection, put them on the shelf in the steam-air sterilization cabinet, set the sterilization temperature to 121℃, sterilize for 10 minutes, and test the sodium hyaluronate solution under these conditions The swelling force value is 0.12MPa, the internal chamber pressure parameter in the back pressure program of the sterilization cabinet is set to 0.16MPa, and the same batch of 200 sodium hyaluronate injections is taken for automatic control sterilization.
Embodiment 2
[0020] Take 200 bottles of 0.5ml:7mg sodium hyaluronate injection, put them on the shelf in the steam-air sterilization cabinet, set the sterilization temperature to 123℃ and sterilize for 10 minutes, and test the sodium hyaluronate solution under these conditions The expansion force value is 0.14MPa, the internal chamber pressure parameter in the back pressure program of the sterilizer is set to 0.19MPa, and another 200 pieces of sodium hyaluronate injection from the same batch are taken for automatic control sterilization.
Embodiment 3
[0022] Take 200 bottles of 2.5ml: 25mg sodium hyaluronate injection, put them on the shelf in the steam-air sterilization cabinet, set the sterilization temperature to 126℃ for 10 minutes, and test the sodium hyaluronate solution under these conditions The swelling force value is 0.13MPa, the internal chamber pressure parameter in the back pressure program of the sterilizer is set to 0.18MPa, and another 200 sodium hyaluronate injections of the same batch are taken for automatic control sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com