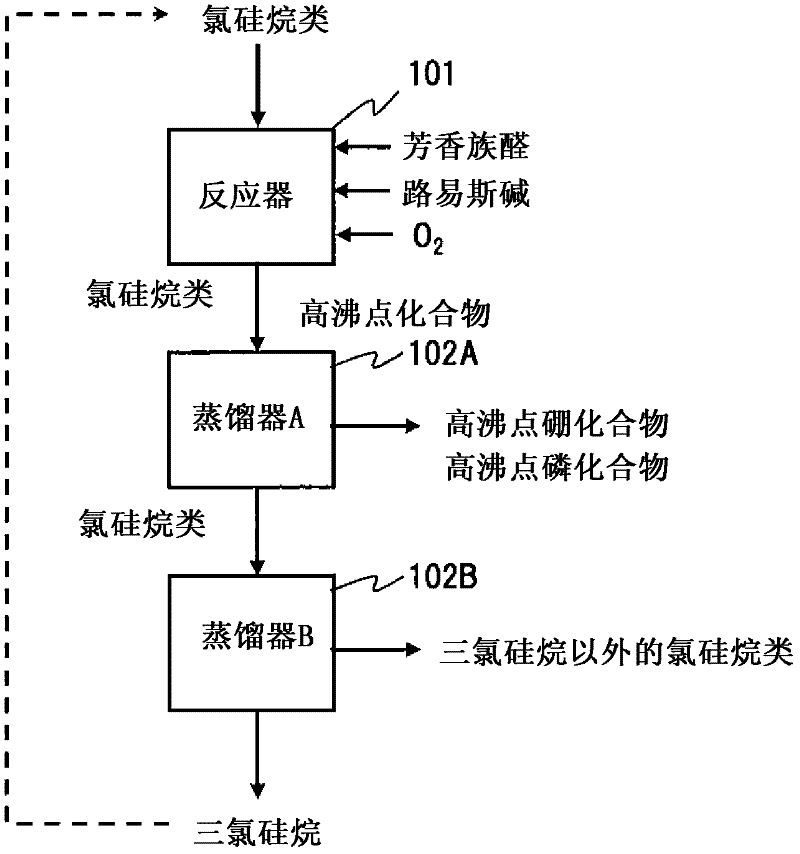
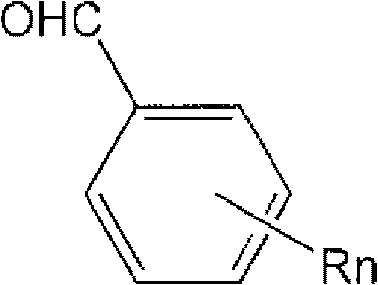
Method for purifying chlorosilanes
A purification method and technology of chlorosilanes, which are applied in the direction of halosilanes, chemical instruments and methods, silicon compounds, etc., can solve the problems of impracticality, reduced concentration rate, and increased discarding volume, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] In order to mask the iron involved in the reaction to form by-product solids, sulfur-containing compounds were added to confirm the effect as an inhibitor of solid by-product formation.
[0089] Specifically, 1 g of PhCHO, PhCH 2 Cl or PhCHCl 2 and 2g of SPh 2 、CH 3 SPh or CH 3 COSPh added to HSiCl 3 (20g), and further add 0.01g of FeCl to it 3 as a catalyst substance. These samples were placed in airtight containers and left to stand at normal temperature for a week to examine the state of solid by-product formation. The results are shown in Table 1.
[0090] Table 1
[0091]
[0092] ○: added ×: not added
[0093] According to the results shown in Table 1, in the above nine samples, after adding PhCHO and SPh 2 The formation of solid by-products was not observed in samples other than . These results show that using sulfur-containing compounds such as CH 3 SPh and SPh 2 effectively inhibits the PhCH 2 Cl and PhCHCl 2 Friedel-Crafts polymerization, whi...
Embodiment 2
[0101] In order to suppress the reaction of forming a by-product solid by polymerization of siloxane, an alkoxysilane was added to confirm the effect as a solid by-product formation inhibitor.
[0102] Specifically, 1 g of PhCHO, PhCH 2 Cl or PhCHCl 2 and 2 g of CH 3 Si(OCH 3 ) 3 or (CH 3 ) 2 Si(OCH 3 ) 2 Add to HSiCl 3 (20g), and further add 0.01g of FeCl to it 3 as a catalyst substance. These samples were placed in airtight containers and left to stand at normal temperature for a week to examine the state of solid by-product formation. The results are shown in Table 3.
[0103] table 3
[0104]
[0105] ○: added ×: not added
[0106] From this experiment, it was found that alkoxysilanes have an effect of inhibiting the polymerization of siloxane, and that alkoxysilanes have no effect of preventing by-product formation of benzylidene polymers, whereas sulfur-containing compounds have this effect.
Embodiment 3
[0114] An experiment for confirming the effect of using a sulfur compound and an alkoxysilane in combination was conducted.
[0115] Specifically, 1 g of PhCHO, PhCH 2 Cl or PhCHCl 2 and 2 g of CH as a sulfur compound 3 SPh and 2 g of CH as an alkoxysilane 3 Si(OCH 3 ) 3 Add to HSiCl 3 (20g), and further add 0.01g of FeCl to it 3 as a catalyst substance. These samples were placed in airtight containers and left to stand at normal temperature for a week to examine the state of solid by-product formation. The results are shown in Table 5.
[0116] table 5
[0117]
[0118] ○: added ×: not added
[0119] Solid by-product formation was not observed in any of the samples, and it was confirmed that the reaction to form a by-product solid could be suppressed by the synergistic effect of each inhibitory effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com