Method for preparing leuprorelin acetate, product and application
A technology of leuprolide acetate and resin, which is applied in the field of medicine, can solve the problems of short service life of fillers, low yield, and high product safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
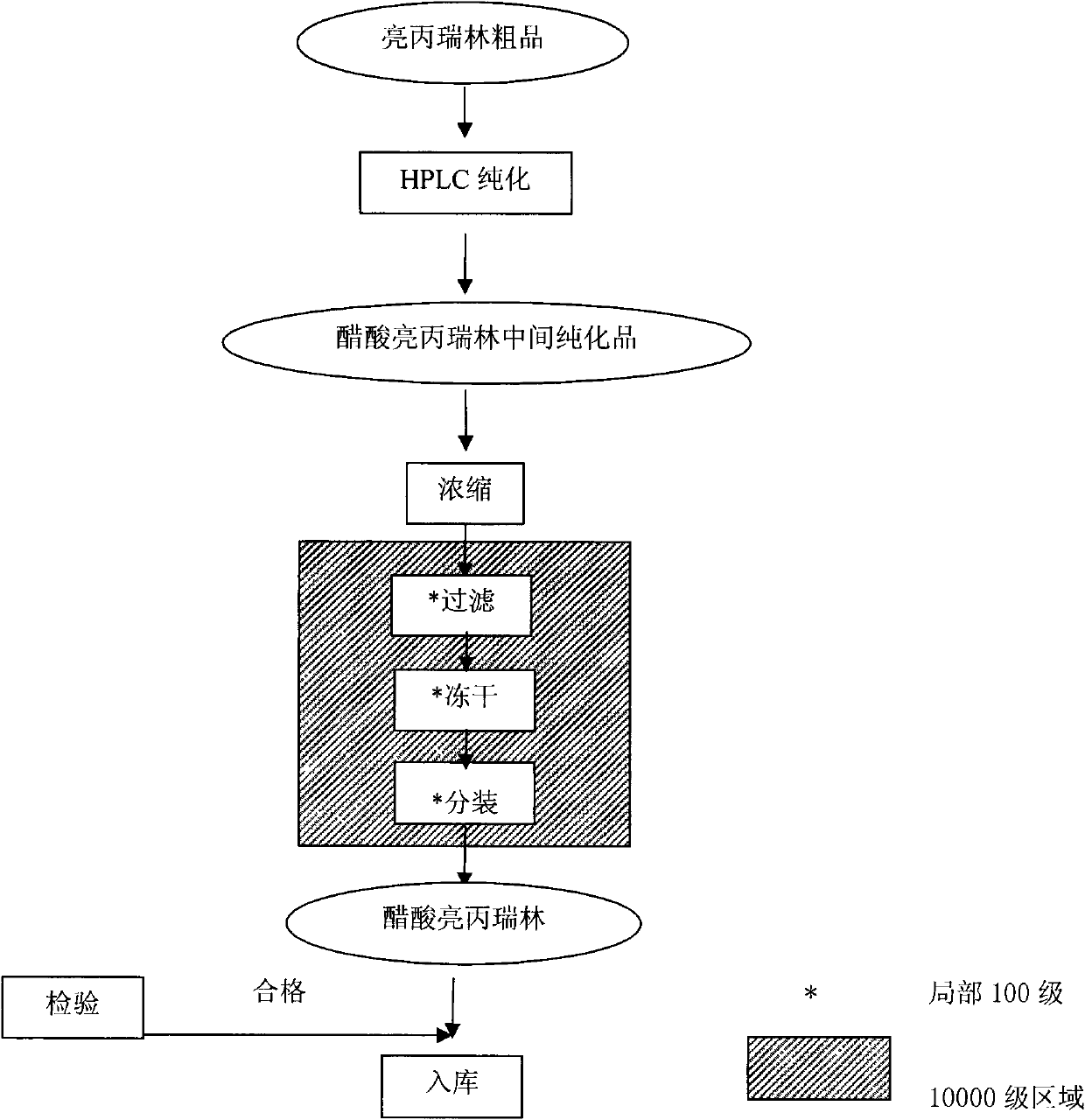
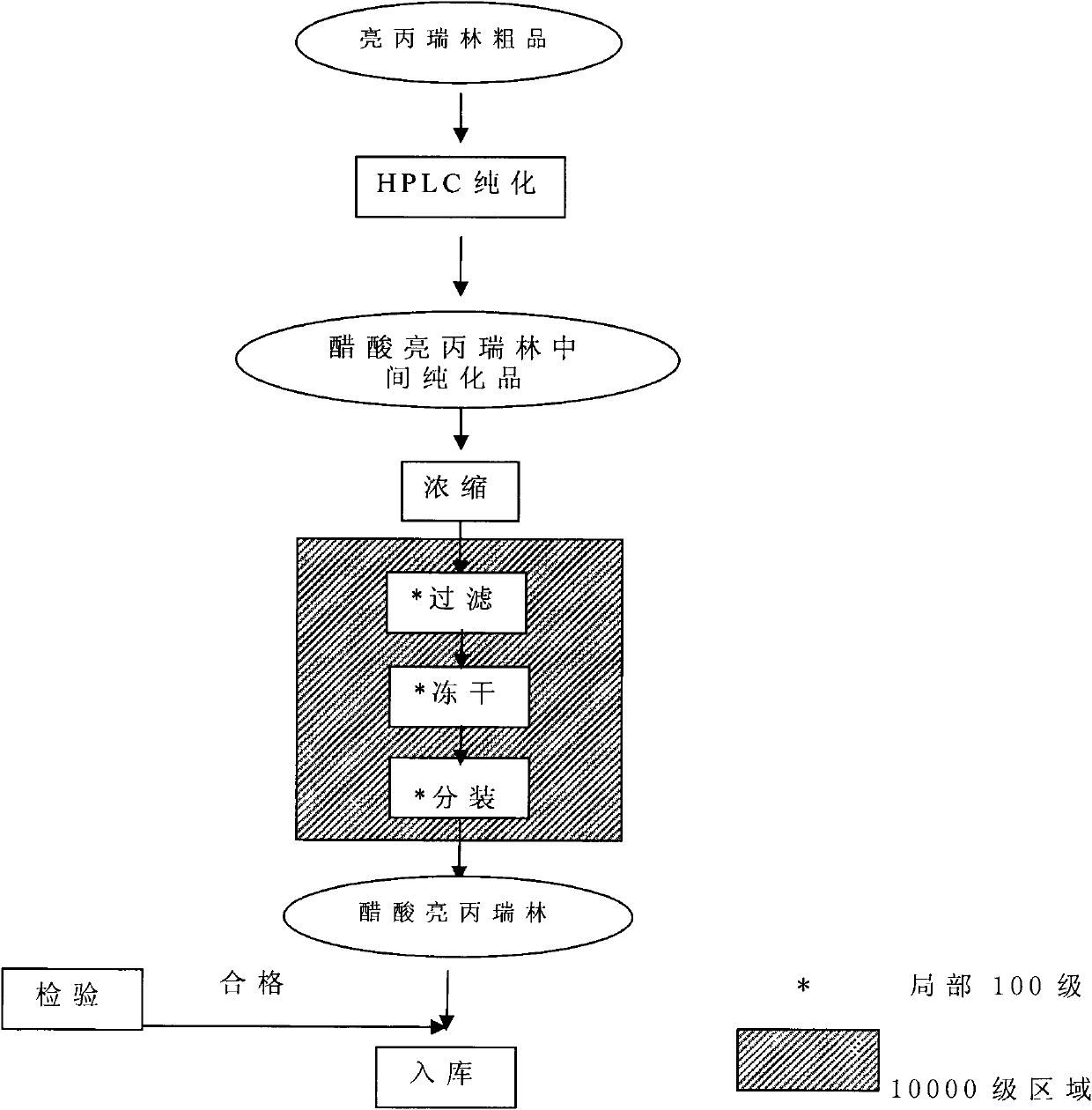
Image
Examples
Embodiment 1
[0066] 1. Preparation of Boc-Pro-
[0067] Weigh 103.2g of Boc-Pro in a 3L three-necked flask and dissolve it in 600ml of methanol. Weigh 101.4g of Cs in a 1L three-neck flask 2 CO 3 Dissolved in 600mlH 2 Slowly add to the above Boc-Pro solution in O to obtain a colorless and transparent Boc-Pro-Cs solution with a pH value of 7.0-7.5; concentrate under reduced pressure in a water bath at 40°C, evaporate the solvent to obtain a solid Add methanol 250ml, evaporate to dryness under reduced pressure, add methanol 250ml and evaporate to dryness under reduced pressure again, in P 2 o 5 Dry under reduced pressure in a desiccator to constant weight to obtain 160-185 g of dried Boc-Pro-Cs.
[0068] Weigh the resin Cl-CH 2 - Put 300g in a 3L Erlenmeyer flask; add the above-mentioned Boc-Pro-Cs into anhydrous DMF solvent, heat to 40°C, pour the Boc-Pro-Cs into the resin after dissolving, then rinse the three-necked flask with anhydrous DMF, Merge into the resin, share 1000ml o...
Embodiment 2
[0162] Example 2 is a comparison of the yield and purity of the leuprolide acetate product prepared by the existing production process with the leuprolide acetate product prepared in Example 1 of the present invention, see Table 3.
[0163] Table 3 existing technology and the leuprolide acetate product that technology of the present invention prepares compares
[0164] Finished product HPLC purity
[0165] As can be seen from Table 3, the HPLC purity of the leuprolide acetate product prepared by the method provided by the invention is high, and the yield of the finished product is also high.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com