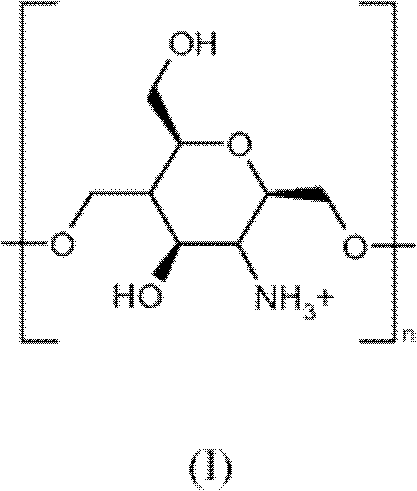
Process for the preparation of colloidal systems for the delivery of active compounds
A technology of colloidal systems and compounds, which is applied in the direction of cosmetic preparations, toiletry preparations, capsule delivery, etc., can solve the problems of not describing the homogenization method of the colloidal system, not proposing the colloidal system, etc., to achieve rapid cost Benefit ratio, increased specific surface area, reduced evaporation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] The oils used in the preparation of the lipophilic phase include plant-based oils, which are understood to be any oils that can be obtained from plant sources. Examples of plant-based oils include sunflower oil, corn oil, soybean oil, cottonseed oil, peanut oil, soybean oil, evening primrose oil, borage oil, olive oil, coconut oil, palm oil, hemp seed oil, almond or seed oil. Seed oil, wheat germ oil, corn germ oil, cocoa butter, macadamia oil, almond oil, avocado oil, calendula oil, grape seed oil, sesame oil, jojoba oil or hazelnut oil.
[0074] However, in the context of the present invention, the term oil is also intended to include oils with one or more unsaturated and / or saturated chain fatty acids or derivatives thereof. Examples of the fatty acids include, but are not limited to, saturated fatty acids such as caprylic / capric triglycerides (Mygliol), lauric acid, myristic acid, palmitic acid, stearic acid, acacia acid (acacidic acid), carnacilic acid, araquidic acid...
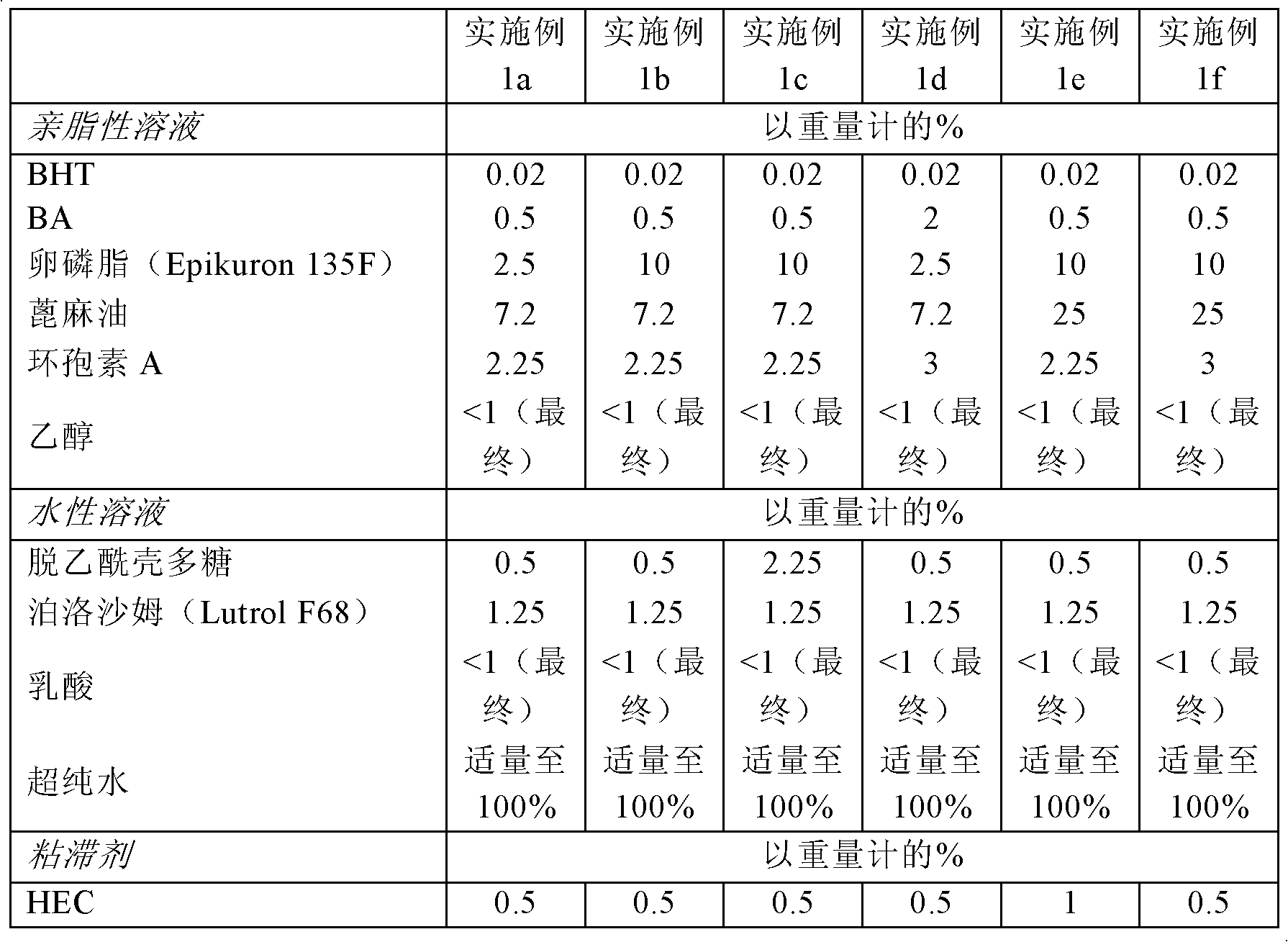
Embodiment 1
[0144] Example 1. Preparation of chitosan nanocapsules containing cyclosporin A
[0145] Using different ratios of phospholipid component (Epikuron), oil (castor oil), chitosan and active ingredient (cyclosporin, CyA), nanocapsules were prepared according to the steps of the present invention. Batches between 0.5L and 5L were prepared.
[0146] The common steps used to prepare nanocapsules are as follows:
[0147] 1. Preparation of lipophilic phase
[0148] First, using a mechanical overhead mixer and a heating plate at a temperature of 50°C, dissolve the corresponding amounts of lecithin (Epikuron 135F), dibutylhydroxytoluene (BHT), benzyl alcohol (BA) and castor oil under mechanical stirring. Stainless steel reaction flask.
[0149] Then, cyclosporine and ethanol were slowly added to the above solution while the temperature was increased to 80°C. Continue stirring until all components are dissolved and part of the ethanol is removed.
[0150] 2. Preparation of the aqueous phase
[0...
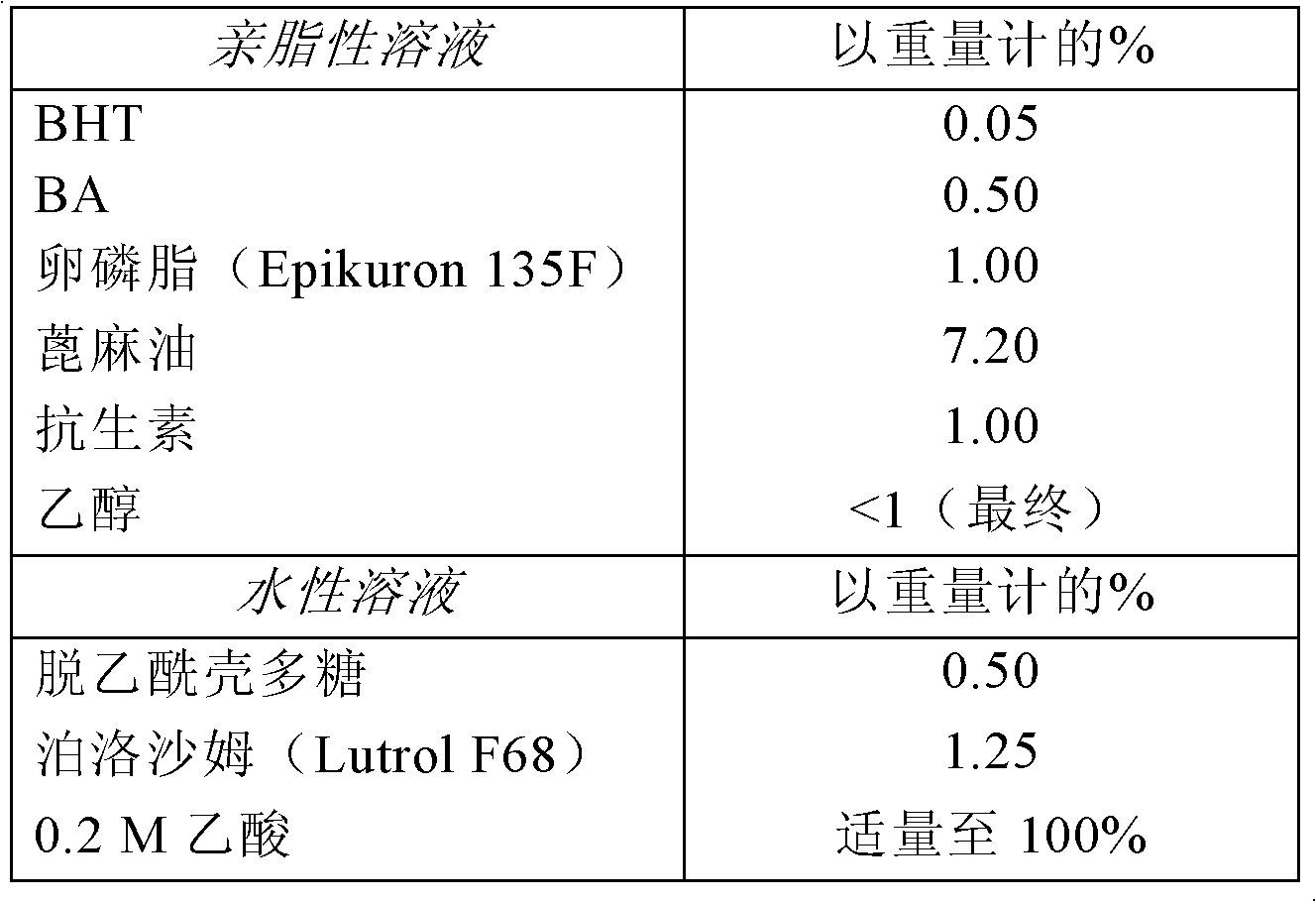
Embodiment 2
[0161] Example 2. Preparation of chitosan nanocapsules containing antibiotics
[0162] The active molecules used in this preparation are macrolide antibiotics such as erythromycin, clarithromycin, azithromycin, roxithromycin, and dirithromycin.
[0163] 1. Preparation of lipophilic phase
[0164] First, using magnetic stirring at 50°C, 6.0 g of active molecules and 0.3 g of dibutylhydroxytoluene (BHT) are dissolved in a container containing 3.0 g of benzyl alcohol and 6 ml of ethanol. Then add 6.0 g lecithin (Epikuron 135F) and 43.2 g castor oil to the above solution and continue stirring until all the components are dissolved.
[0165] 2. Preparation of the aqueous phase
[0166] The aqueous phase was prepared by dissolving 3.0 g of chitosan, 7.5 g of poloxamer, and 531.0 g of 0.2 M acetic acid in a container by using magnetic stirring and heating on a hot plate at a temperature of 50°C.
[0167] 3. Emulsion processing
[0168] By using the Turrax emulsifier at the highest stirring spe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com