Crystal form of oxalate of dipeptidyl peptidase inhibitor and preparation method and application thereof
A technology of dipeptidyl peptidase and cyanopyrrolidine oxalate, which is applied in the field of new crystal forms of medicines, can solve the problems of unimproved stability of the compound of formula 1, poor compound stability, compound stability problems and the like, To achieve the effect of product safety assurance, easy storage and transportation, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 Preparation of crystal form of the present invention
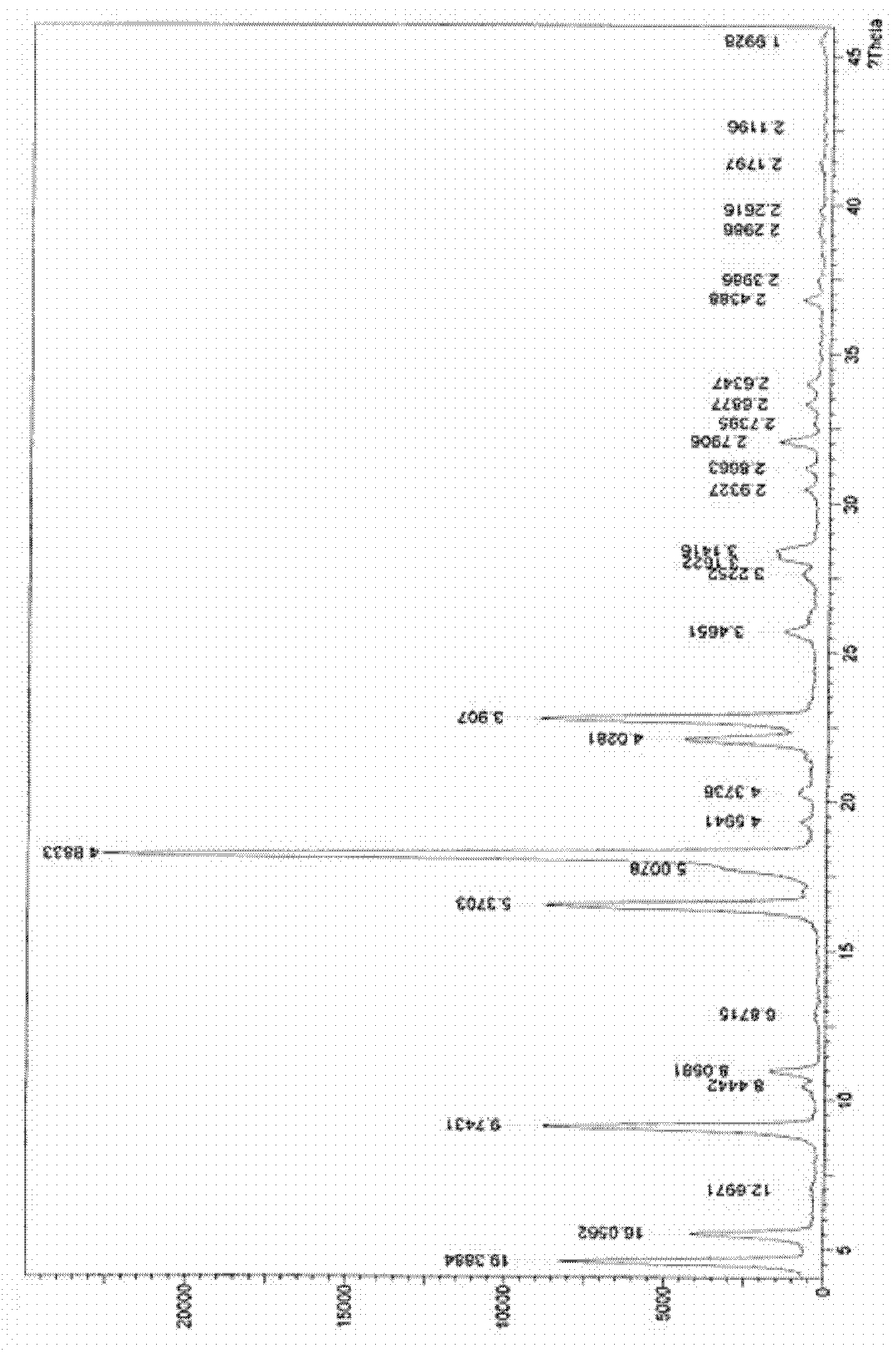
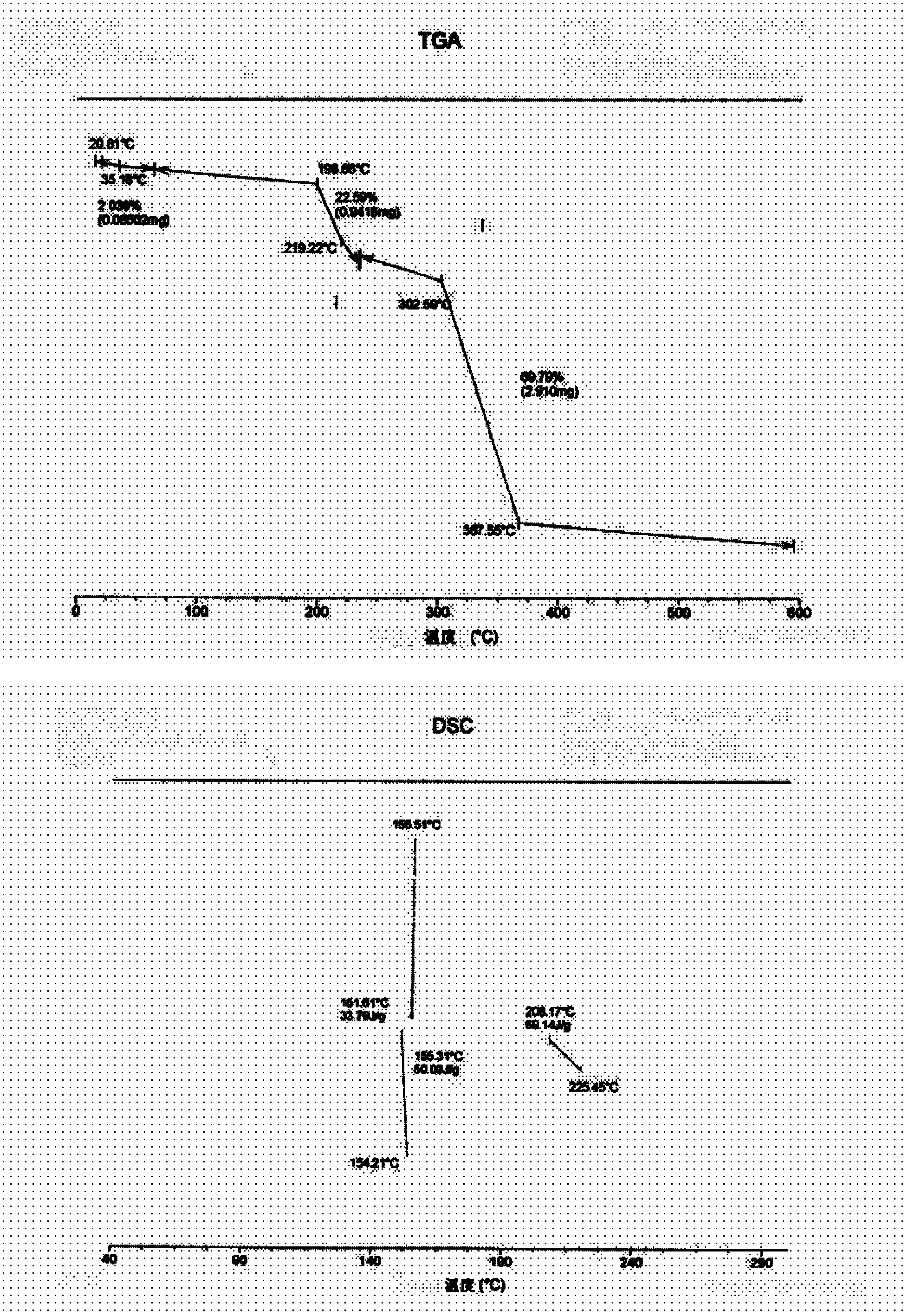
[0062] Take (s)-1-(2-(2-(3-(3,4-dimethoxyphenyl)-2-oxo-1-imidazolidinyl)ethylamino)acetyl)-2- Add 30g of cyanopyrrolidine oxalate and 600g of anhydrous methanol into a 2000ml round bottom flask with a reflux device, heat and reflux in a water bath until completely dissolved, then cool to room temperature, a large number of white flaky crystals precipitate, filter, and put in an oven at 50°C After drying, 28.5 g of the product was obtained, the purity (HPLC) was 99.5%, and the melting point was 154°C-156°C. The endothermic transition is at about 151°C, the results of thermogravimetric-differential thermal analysis are shown in the attached image 3 .
[0063] X-ray powder diffraction results are as follows:
[0064]
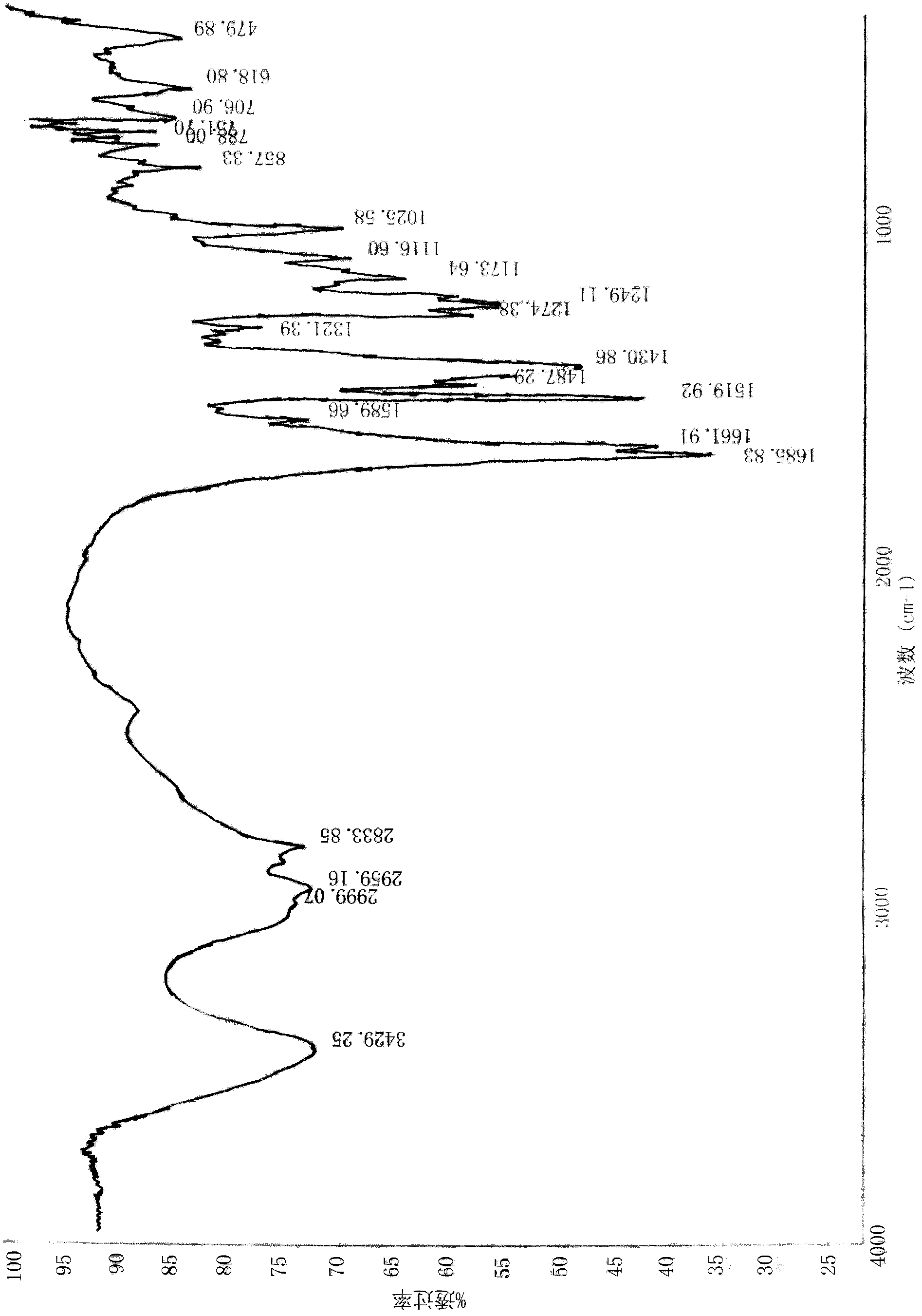
[0065] Infrared spectrum wave number is (cm -1 )为3429.25;2999.07;2959.16;2833.85;1685.83;1661.91;1589.66;1519.92;1487.29;1430.86;1321.39;1274.38;1249.11;1173.64;1116.60;1025.58;857.3...
Embodiment 2
[0080] Embodiment 2 Preparation of crystal form of the present invention
[0081] Take (s)-1-(2-(2-(3-(3,4-dimethoxyphenyl)-2-oxo-1-imidazolidinyl)ethylamino)acetyl)-2- Add 20g of cyanopyrrolidine oxalate and 320g of methanol containing 5% water into a 1000ml round bottom flask with a reflux device, heat and reflux in a water bath until completely dissolved, then cool to room temperature, a large number of white flaky crystals are precipitated, filtered, 50 Oven-dried at ℃ to obtain 18.5 g of the product with a melting point of 155°C-156°C and a purity (HPLC) of 99.5%.
[0082] X-ray powder diffraction results are as follows
[0083] 2θ
[0084] 4.5
[0085] 5.6
[0086] 9.1
[0087] 10.8
[0088] 16.5
[0089] 17.8
[0090] 18.2
[0091] 22.0
[0092] 22.6
[0093] 25.7
[0094] 28.2
[0095] 28.4
[0096] 32.1
Embodiment 3
[0097] Embodiment 3 Preparation of crystal form of the present invention
[0098] Take (s)-1-(2-(2-(3-(3,4-dimethoxyphenyl)-2-oxo-1-imidazolidinyl)ethylamino)acetyl)-2- 20 g of cyanopyrrolidine oxalate and 300 grams of methanol containing 10% water were added into a 1000 ml round-bottomed flask with a reflux device, heated under reflux in a water bath until completely dissolved, then cooled to room temperature, a large amount of white flaky crystals were precipitated, filtered, Oven-dried at 50°C to obtain 18.5g of product with a melting point of 155.5°C-157°C and a purity (HPLC) of 99.5%.
[0099] X-ray powder diffraction results are as follows:
[0100] 2θ
[0101] 4.6
[0102] 5.6
[0103] 9.1
[0104] 10.9
[0105] 16.3
[0106] 17.8
[0107] 18.1
[0108] 22.0
[0109] 22.6
[0110] 25.5
[0111] 28.1
[0112] 28.5
[0113] 32.0
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com