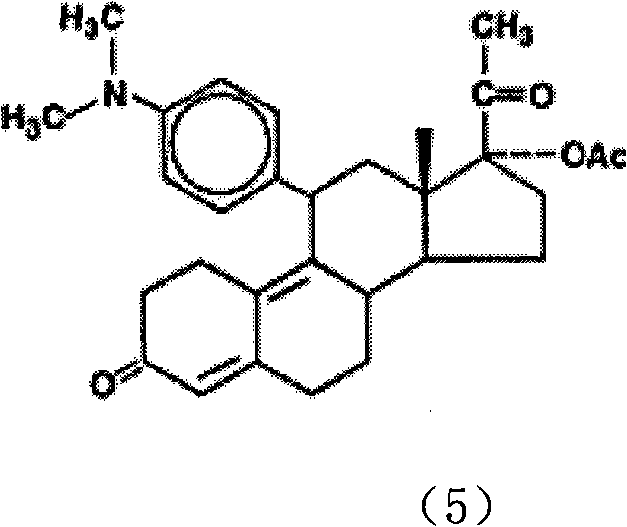
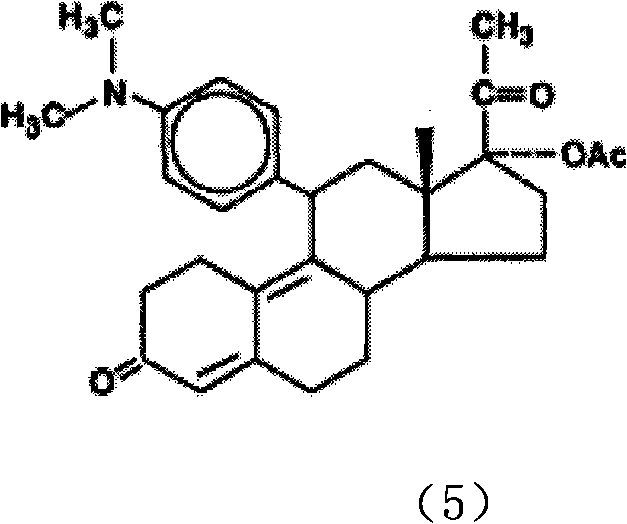
Synthesis method of progesterone receptor regulating agent ulipristal
A synthetic method, Uliplast-style technology, applied in the field of progesterone receptor modulator Uliplast acetate, can solve the problems that affect the purity of the product, and achieve the effect of easy operation, high purity, and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
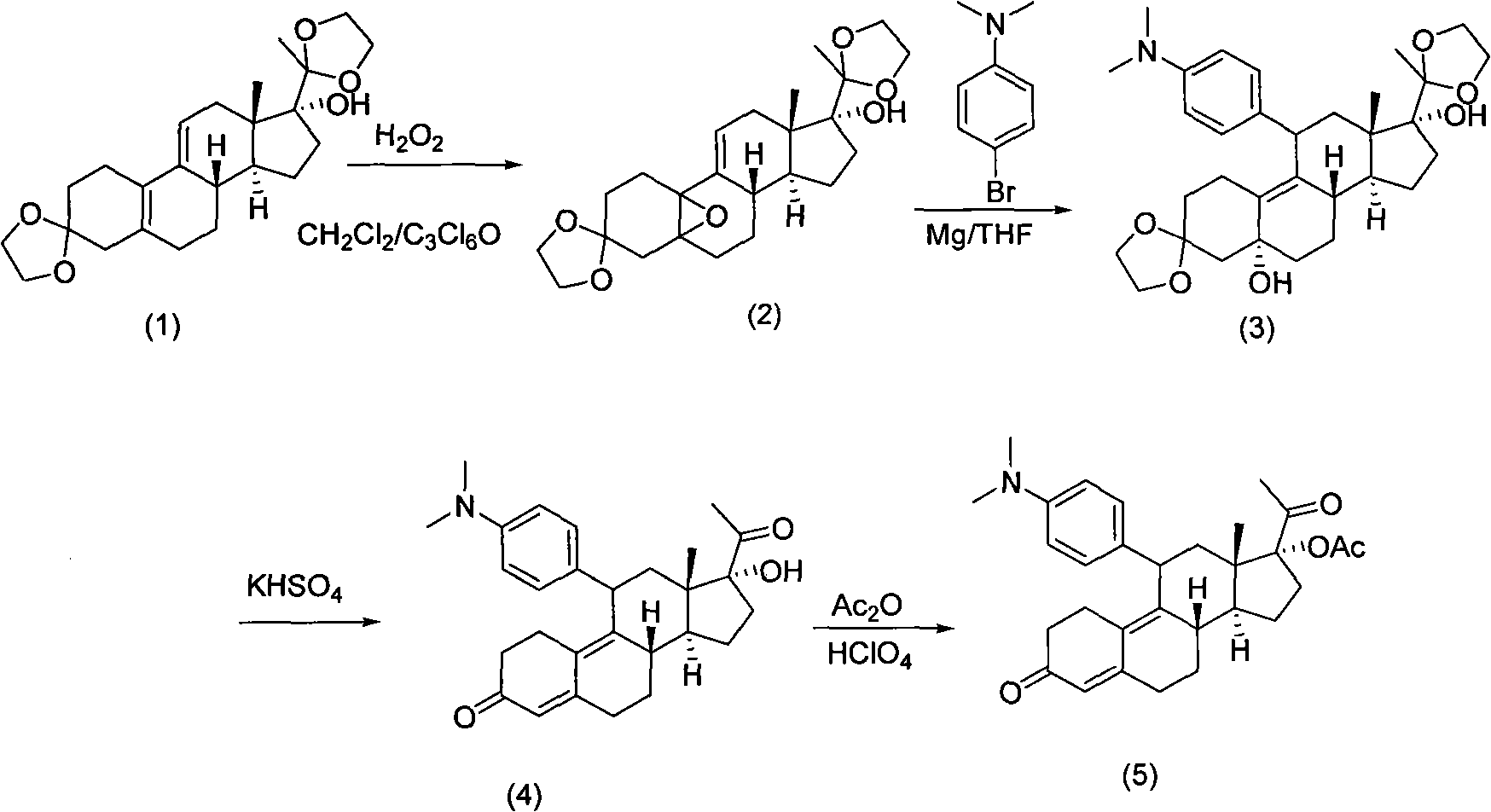
[0015] The preparation of compound (2):
[0016] Dissolve 450 g of the double-protected compound (1) in 4300 ml of dichloromethane, mechanically stir to dissolve, lower the temperature to 0-4° C., add 12 ml of pyridine and 43 ml of hexachloroacetone to the solution, and add 30 ml of hexachloroacetone dropwise at 0-4° C. % hydrogen peroxide 643ml, continue to stir and react at 0-4°C for about 18-24 hours after adding. The basic reaction of the raw materials is complete (developing agent: petroleum ether / ethyl acetate, namely PE / EA=3 / 1, using 5% phosphomolybdic acid ethanol solution as the color developer), and then add 7715ml of dichloromethane, and pour the reaction solution into an ice-cold In the sodium thiosulfate solution (720g sodium thiosulfate / water 6000ml), mechanically stir for 30 minutes at 0-10°C after the addition is complete. Separation, the aqueous layer was extracted with 2140ml of dichloromethane, the combined organic layer was washed with water (2140ml*2), th...
Embodiment 2
[0018] The preparation of compound (3):
[0019] 1130 g of N.N-dimethyl-p-bromoaniline was dissolved in 5656 ml of dry tetrahydrofuran. Add 187.5g of magnesium, 460ml of tetrahydrofuran solution and a small amount of iodine (half a grain) into a 10L three-necked flask, and then add 184ml of the above-mentioned N.N-dimethyl-p-bromobenzene solution in tetrahydrofuran. Heating to 55°C-65°C, the iodine color fades, the solvent refluxes violently, and Grignard triggers. Lower the temperature to an internal temperature of 30-35°C, add the remaining N.N-dimethyl-p-bromoaniline tetrahydrofuran solution dropwise, and finish dropping in about 4 hours, while maintaining an internal temperature of 30-35°C during the dropping process. After dropping, keep the reaction at 30-35°C for 1-2 hours, then cool down to room temperature to obtain the Grignard reagent. In a 10L three-necked flask, 480g of epoxy compound (2), 25.4g of cuprous chloride, and 1450ml of dry THF were added. The solutio...
Embodiment 3
[0021] Preparation of compound (4):
[0022] Potassium bisulfate 127g was dissolved in 1410m water, cooled to 5°C, solid Grignard product 188g compound (3) (PH about 1) was added, the reaction was maintained at 5°C and stirred for 4-5 hours, and the reaction was complete (developing agent: PE / EA = 2 / 1, with ultraviolet color development), after adding 1100ml of dichloromethane and potassium hydroxide aqueous solution (potassium hydroxide 19.4g / water 172ml), stirred for 15 minutes (PH is about 3), separated, and the water layer was used Dichloromethane (940ml*3) was extracted, the organic layer was washed with water (1330ml*2) until neutral, and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to about 480ml of compound (4), which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com