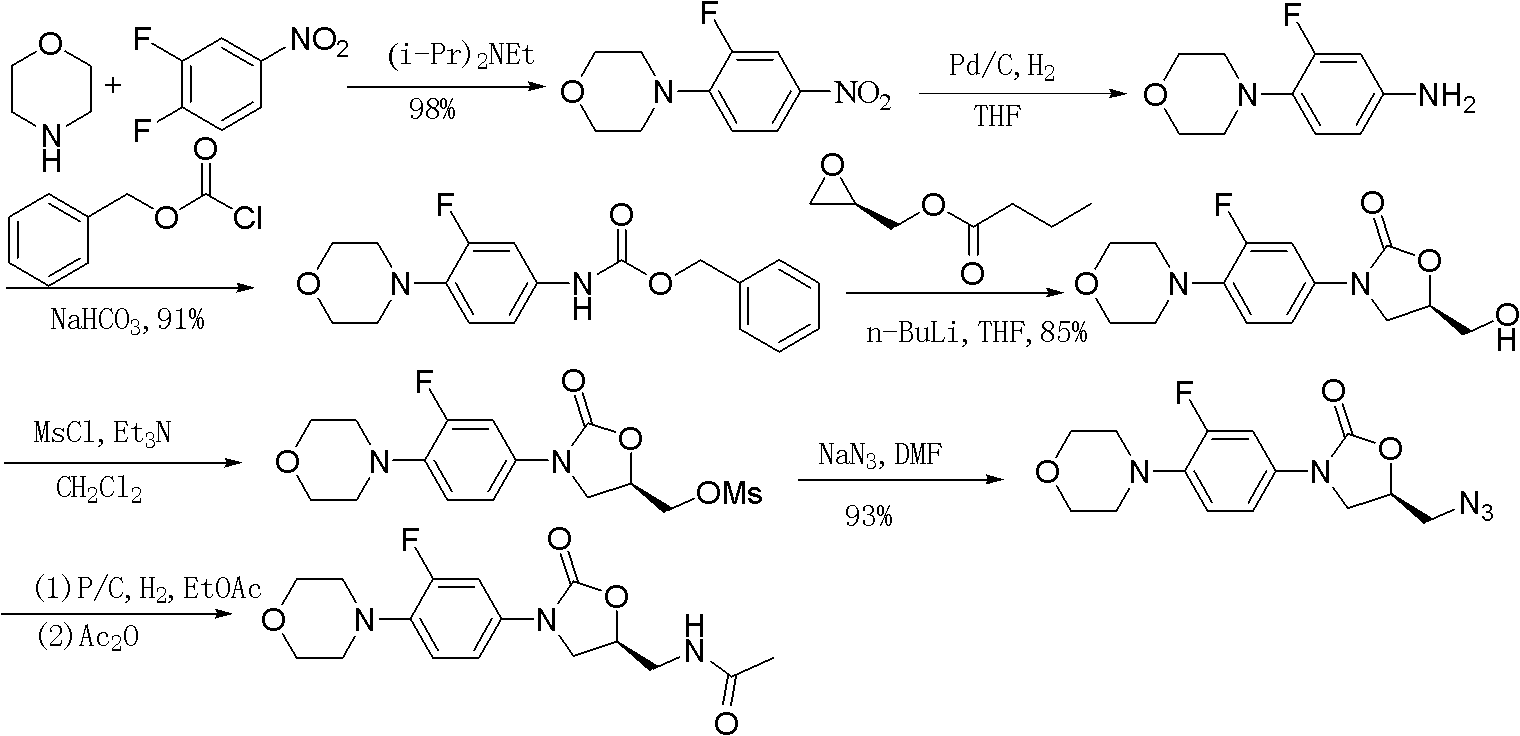
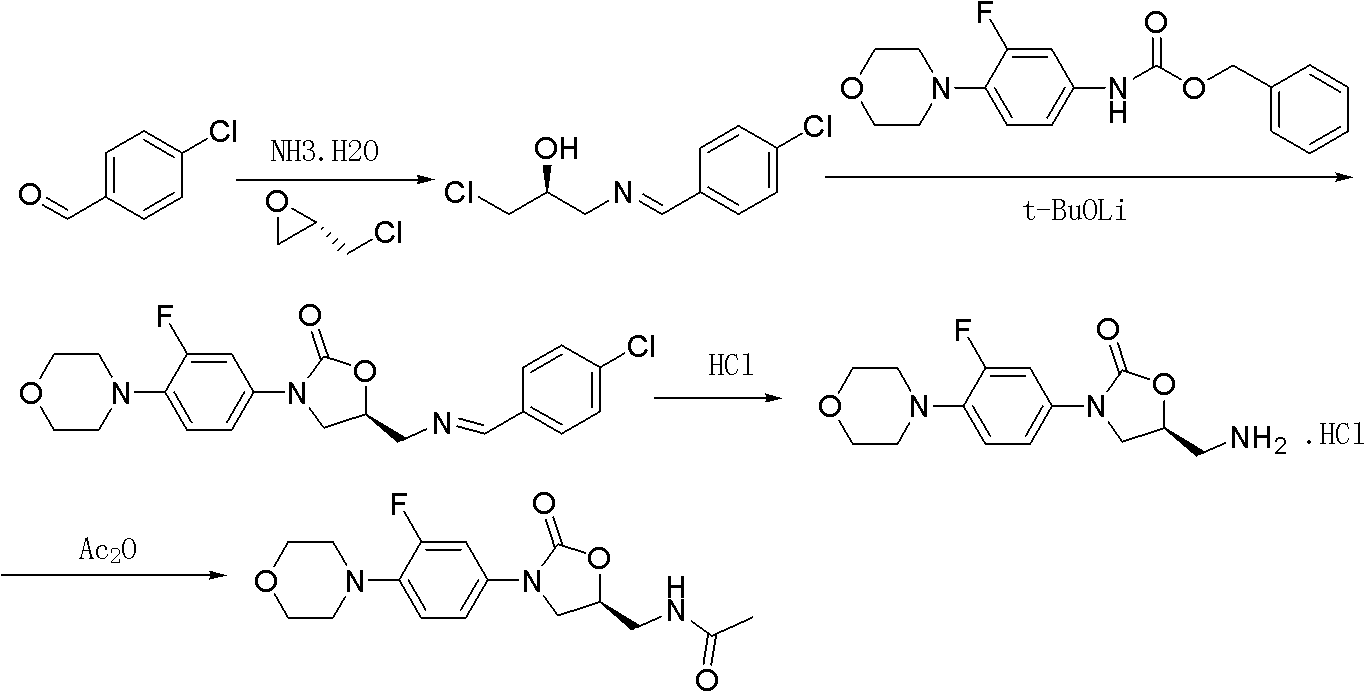
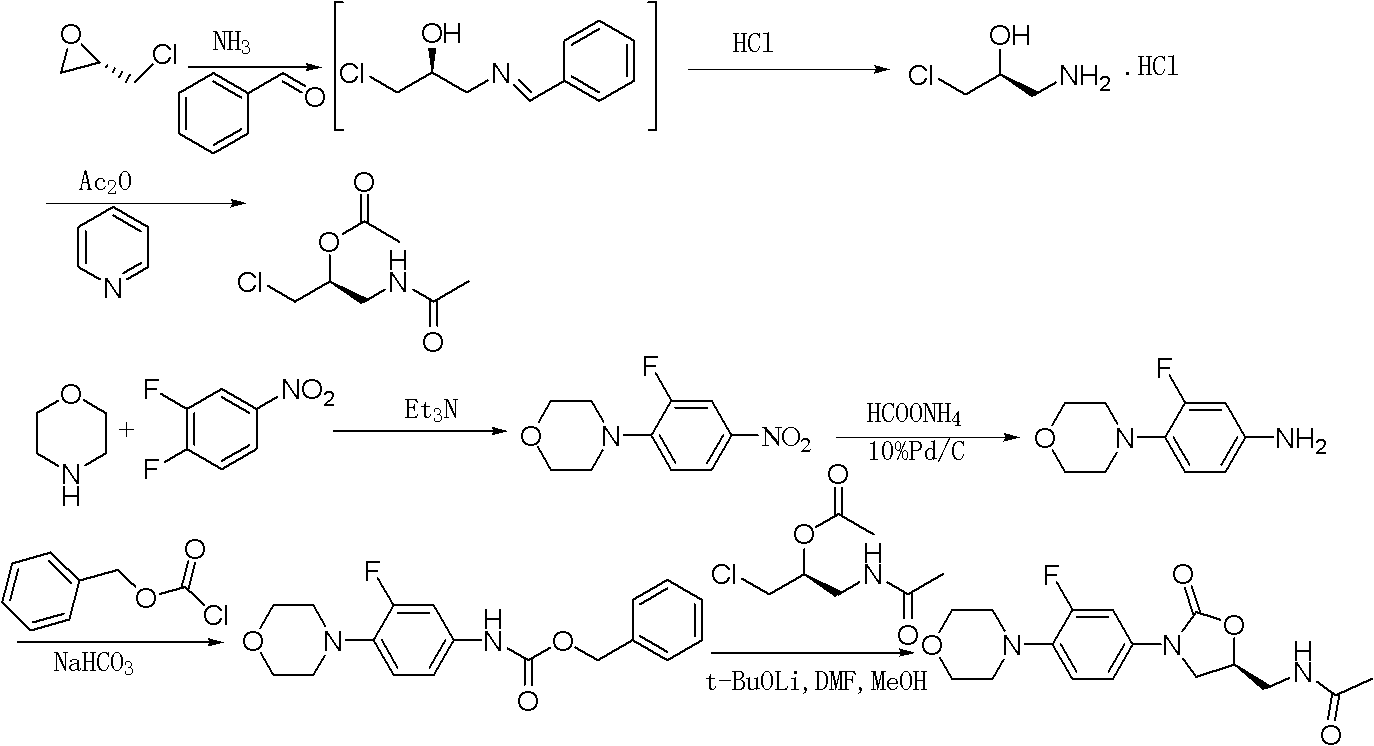
Preparation method of linezolid
A technology of linezolid and oxazolidine, which is applied in the field of pharmacy, can solve the problems of serious environmental pollution, low yield, unfavorable industrial production of linezolid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. Preparation of S-1-azido-3-chloro-2-propanol:
[0049] Add S-epichlorohydrin (50g, 0.543mol) and ammonium chloride (14.5g, 0.272mol) into 200mL of ethanol and 50mL of water, stir for 5min, add sodium azide (37.1g, 0.571mol), and react at room temperature After 48 hours, distill off ethanol, add a large amount of water to dissolve the inorganic salt, extract with dichloromethane, distill off the yellow liquid S-1-azido-3-chloro-2-propanol (51.5 g, 70%).
Embodiment 2
[0050] Example 2. Preparation of 3-fluoro-4-morpholine nitrobenzene:
[0051] Morpholine (5.84mL, 0.066mol) and potassium carbonate (4.5g, 0.033mol) were added to 20mL of ethanol, and 3,4-difluoronitrobenzene (10g, 0.063mol) was added dropwise at room temperature. To reflux, the reaction was complete after 3 hours, the reaction was stopped, and a large amount of solids precipitated out at room temperature, which was added to 50 mL of water, stirred and filtered, and dried to obtain the product 3-fluoro-4-morpholine nitrobenzene (13.8 g, 97%).
Embodiment 3
[0052] The preparation of embodiment three.3-fluoro-4-morpholine aniline:
[0053] Add 3-fluoro-4-morpholine nitrobenzene (11.3 g, 0.05 mol) to a mixed solution of 23 mL of ethanol and 3 mL of water, then add ammonium chloride (1.34 g, 0.025 mol) and glacial acetic acid (1.15 mL, 0.01mol), stir and heat, add iron powder (14g, 0.25mol) in batches when the temperature reaches 70°C, reflux reaction for 3-4h after the addition is complete, the reaction is complete by TLC, stop the reaction, heat filter, filter cake with 12mL Ethanol was refluxed for 0.5h, then hot filtered, the ethanol solution was combined, activated carbon decolorized, most of the ethanol was evaporated, filtered, the filter cake was washed with a small amount of ethanol, and dried to obtain the product 3-fluoro-4-morpholineaniline (7.35g, 75 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com