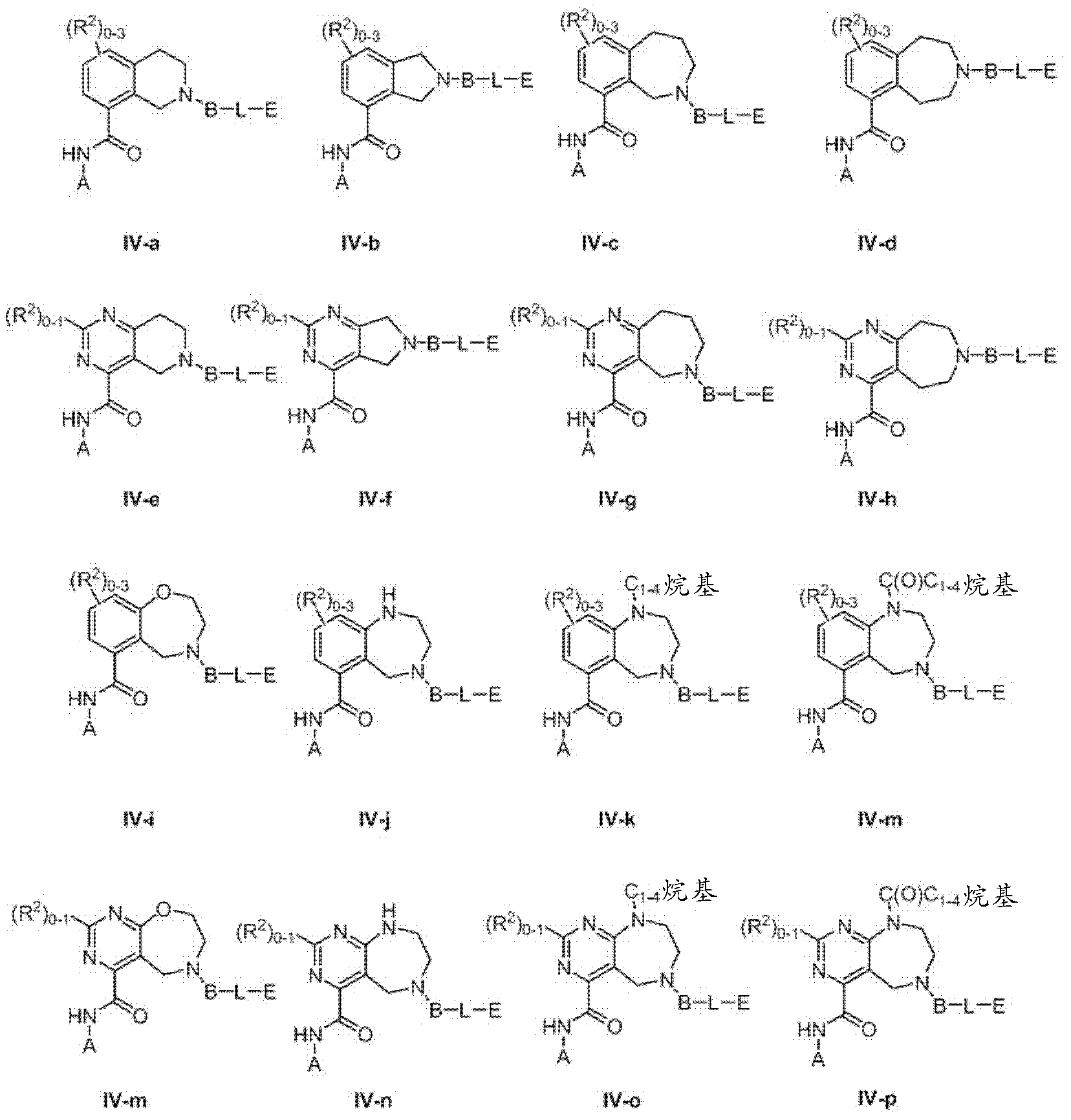
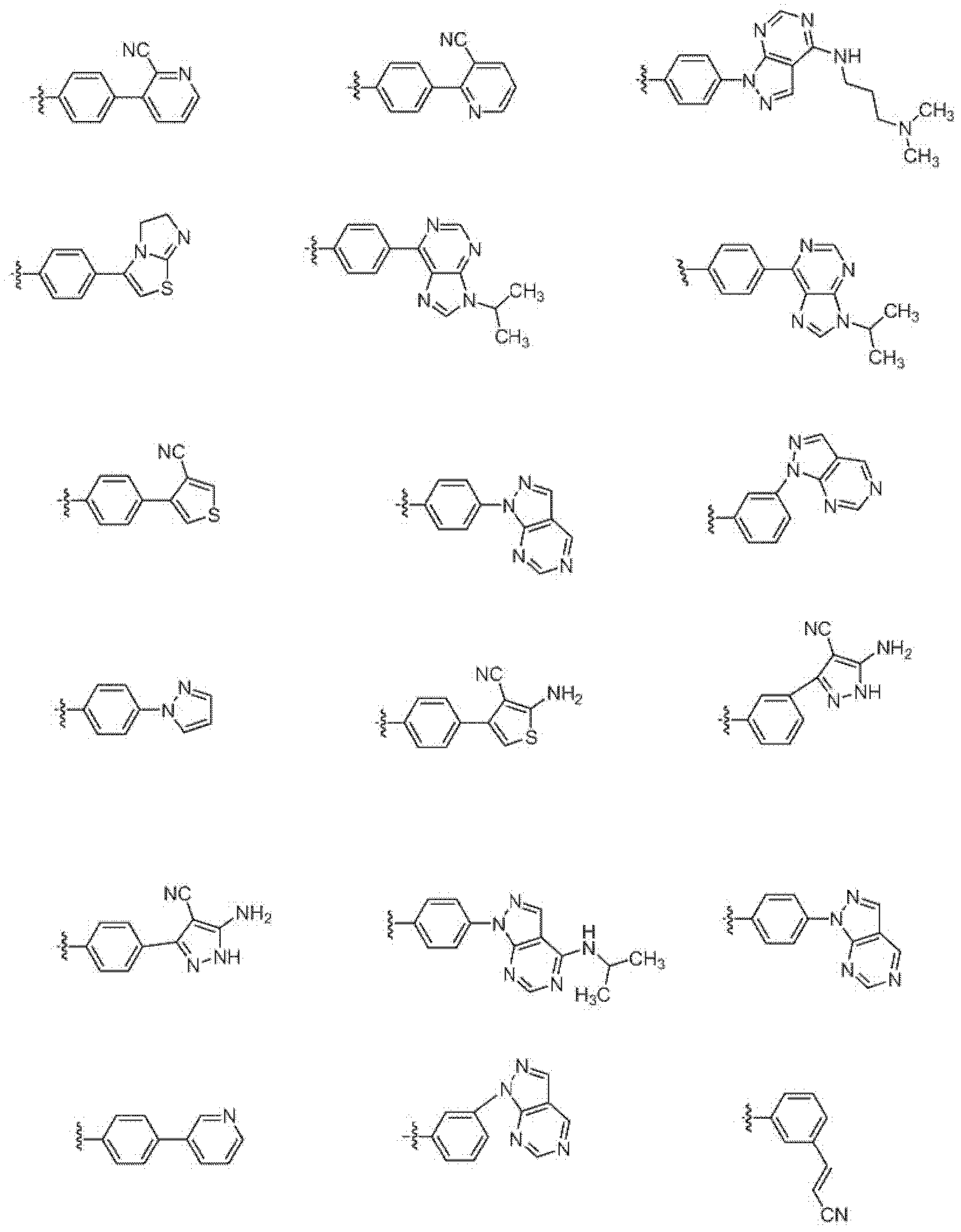
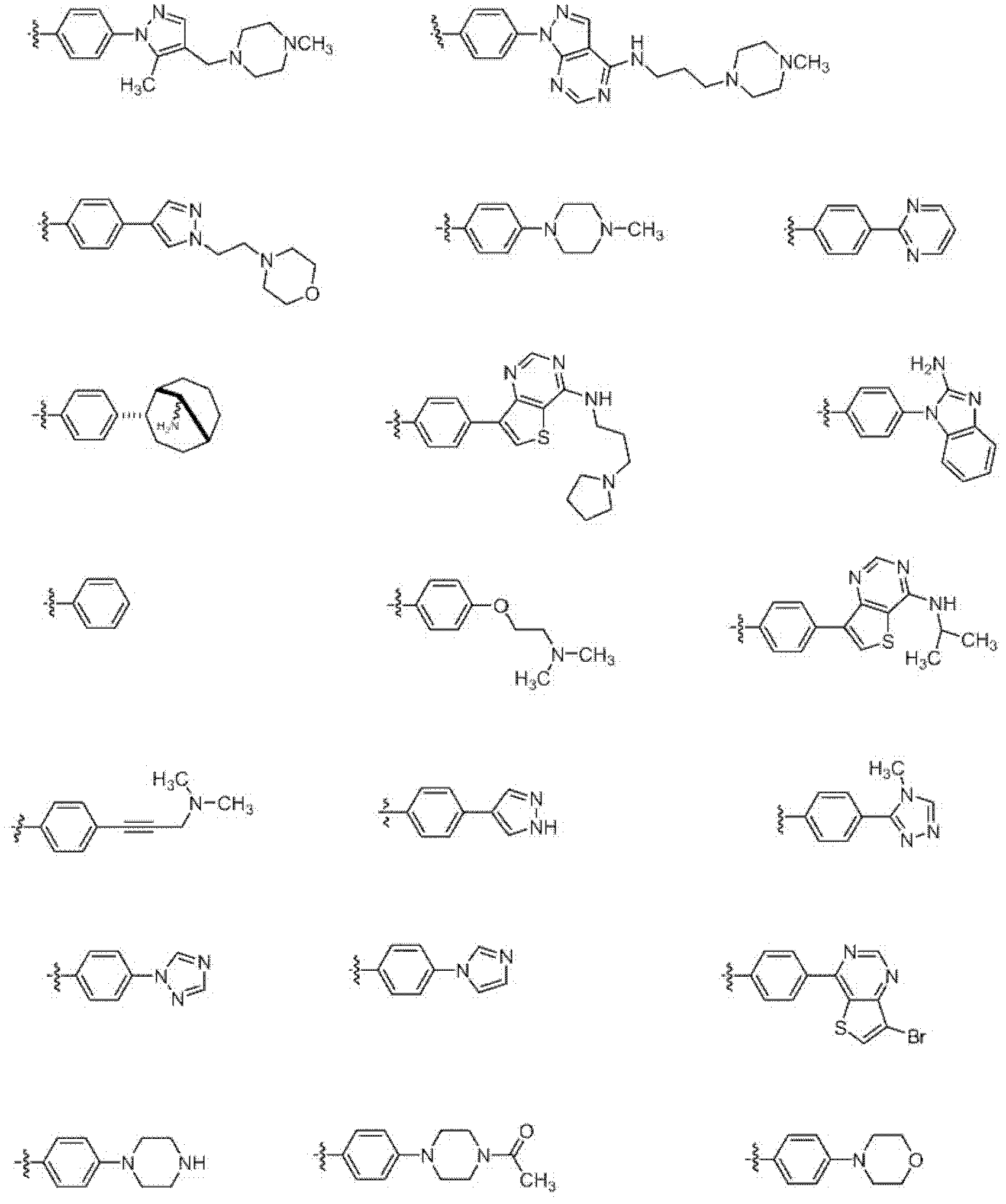Heterocyclic compounds and methods of use
A compound and heterocycle technology, applied in the fields of drug combination, organic chemistry, pharmaceutical formulation, etc., can solve the problems of cytotoxicity, weak binding, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0284] Synthesis of 2-(8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)thiazole-4-carboxylic acid (1):
[0285]
[0286] step 1 : Preparation of tert-butyl 8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate (1A).
[0287]
[0288] To 2-(tert-butoxycarbonyl)-1,2,3,4-tetrahydroisoquinoline-8-carboxylic acid (6.8g, 24.5mmol) and benzo[d]thiazol-2-amine (5.52g , 36.8 mmol) in DCM (80 mL), EDCI (9.4 g, 49.04 mmol) and DMAP (6 g, 49 mmol) were added. The mixture was stirred overnight at room temperature. The reaction mixture was diluted with DCM (400 mL), washed with 5% aqueous HCl, water, brine, washed with Na 2 SO 4 Drying and concentration under reduced pressure afforded 8.5 g of the desired product 8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinoline-2(1H)-tert-butylcarboxylate Esters (1A): 1 H NMR (300MHz, CDCl 3 )δppm 7.83 (1H, m), 7.48 (1H, d), 7.34 (4H, m), 7.19 (1H, t), 4.91 (2H, m), 3.67 (2H, t), 2.92 (2H, t) ,...
Embodiment 2
[0298] 2-(8-(Benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)-5-(3-phenoxypropyl) Synthesis of thiazole-4-carboxylic acid (2):
[0299]
[0300] step 1 : Preparation of ethyl 3-bromo-6-chloro-2-oxohexanoate (2A).
[0301]
[0302] Bromine (0.85 mL, 16.5 mmol) was added to carbon tetrachloride (30 mL) containing ethyl 6-chloro-2-oxohexanoate (2.9 g, 15 mmol), and stirred at room temperature for 1 hour. The reaction mixture was diluted with EtOAc, washed with Na2 S 2 o 3 solution, water, brine, washed with MgSO 4 Dry, filter and concentrate under reduced pressure. The crude material was purified by column chromatography on silica gel eluting with a gradient of 0 to 10% EtOAc in hexanes to afford the desired product ethyl 3-bromo-6-chloro-2-oxohexanoate (2A) in 95% yield %. 1 H NMR (300MHz, DMSO-d 6 ) δ ppm 5.25 (1H, dd), 4.29 (2H, q), 3.71 (2H, t), 2.16 (1H, m), 1.91 (1H, m), 1.29 (3H, t).
[0303] step 2 : 2-(8-(Benzo[d]thiazol-2-ylcarbamoyl)-3,...
Embodiment 3
[0312] 2-(8-(Benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)-5-(3-(pyridin-4-yl Synthesis of thio)propyl)thiazole-4-carboxylic acid (3):
[0313]
[0314] The title compound 2-(8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinolin-2(1H)-yl)-5-(3-(pyridine- 4-ylthio)propyl)thiazole-4-carboxylic acid (3) was prepared by the following procedure: The title compound was prepared by substituting pyridine-4-thiol for phenol in step 4 of Example 2. After precipitation of the desired product, the solid was purified by HPLC (pre-preparative reverse phase HPLC performed with an automated Gilson HPLC system using a SymmetryPrep Shield RP18 pre-preparative column, 250mmx21.20mm i.d., 10um, flow rate 25mL / min; λ = 214, 245nm; flow Phase A, H with 0.1% TFA 2 O; mobile phase B, CH 3 CN; linear gradient 0-90% of B in 40 minutes) to provide 2-(8-(benzo[d]thiazol-2-ylcarbamoyl)-3,4-dihydroisoquinoline-2 (1H)-yl)-5-(3-(pyridin-4-ylthio)propyl)thiazole-4-carboxylic acid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com