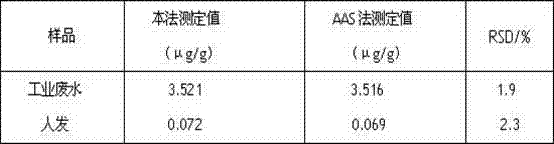
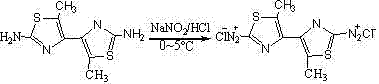2,2'-bis(2-hydroxy-4-sulfonic-1-naphthylamine azoxyl)-5,5'-dimethyl-4,4'-bithiazole and preparation method thereof
A naphthylaminoazo and dimethyl technology, which is applied in the field of diheterocyclic triazene compounds, can solve the problems of fluorescence detection of few metal ions, and the selectivity and sensitivity need to be improved, and achieves a simple preparation method and high sensitivity. , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
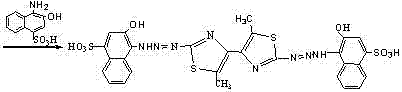
[0038] 2, 2'-bis(2-hydroxy-4-sulfonic acid-1-naphthylaminoazo)-5, 5' - Dimethyl-4, 4' - Bithiazole, its molecular structural formula:
[0039]
[0040] 2, 2'-bis(2-hydroxy-4-sulfonic acid-1-naphthylaminoazo)-5, 5' - Dimethyl-4, 4' - The preparation method of bithiazole comprises the following steps:
[0041] (1) Preparation of 2,5-dibromohexanedione
[0042] Add 28.5g (0.25mol) 3,4-hexanedione, 45mL chloroform and 10mL glacial acetic acid into a 250mL three-neck flask equipped with a magnetic stirrer dropping funnel and a reflux device, stir and heat to about 80°C on an oil bath , Slowly drop the mixture of 80g liquid bromine and 30mL chloroform from the separatory funnel, and the reaction starts immediately. After the bromine is added dropwise, continue heating until the solution turns from brown-red to orange-yellow, then quickly cool it to 0°C in an ice bath, and yellow needle-like precipitates appear, filter with suction, and recrystallize with chloroform to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com