Conjugates of hydroxyalkyl starch and a protein
A technology of hydroxyalkyl starch and conjugates, which is applied in the field of conjugates of hydroxyalkyl starches and proteins, and can solve problems such as not being very clear about the metabolic pathway of non-natural polymer PEG
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0327] Example 1: Synthesis of functionalized HES:
[0328] Example 1.1 Synthesis of Amino-HES from Oxidized HES
[0329] 5.122 g of HES (MW=14,500 D, DS=0.41, Supramol Parenteral Colloids GmbH, Rosbach-Rodheim, D) selectively oxidized at its reducing end groups according to the description of DE 19628705 A1 were heated at 80° C. under vacuum for 15.5 hours And it was dissolved in 25 mL dry DMSO (Fluka, Sigma-Aldrich Chemie GmbH, Taufkirchen, D) under nitrogen. To the resulting solution was added 51.22 mmol of 1,4-diaminobutane (Fluka, Sigma-Aldrich Chemie GmbH, Taufkirchen, D). After it was incubated at 40° C. for 17 hours, the reaction mixture was added to 150 mL of ice-cold 1:1 ethanol (DAB, Sonnenberg, Braunschweig, D) and acetone (Carl Roth GmbH+Co.KG, Karlsruhe, D). (v / v) mixture and incubated at -20°C for 1 hour. The precipitated product was collected by centrifugation at 4°C, washed with 40 ml of the same mixture, redissolved in 80 mL of water, and dialyzed against ...
Embodiment 2
[0336] Example 2 Synthesis of protein-HES conjugates by chemical ligation:
Embodiment 21
[0337] Example 2.1 Synthesis of peptide-thioester A-HES conjugates from H-Cys(S-tBu)-HES and peptide-thioester A
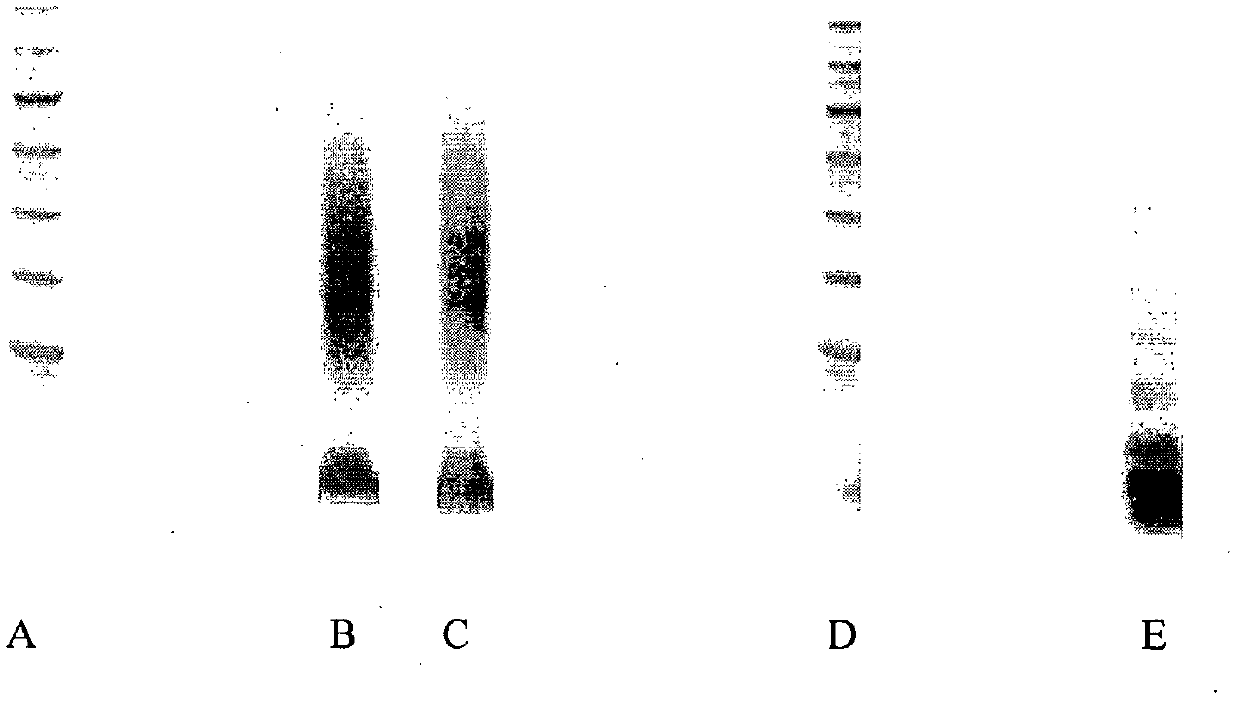
[0338] At 22°C, add 5 μL of 10 mg / mL peptide-thioester A (GBF, Braunschweig, D, amino acid sequence: H-SPFGADTTVCCFNYSVRKLPQNHVKDYFYTSSK-ethyl thiopropionate; MW=3,920 g / mol) at 1:1 ( v / v) N, N-dimethylformamide (peptide synthesis grade, Biosolve, Valkenswaard, NL) and 0.1M sodium phosphate buffer (pH 7.2) in the solution of the mixture, add 5 μ L 255mg / mL as in Example H-Cys(S-tBu)-HES solution synthesized as described in 1.2 and 5 μL of 1.5M catalyst solution in DMF. As a catalyst, thiophenol ( figure 1 , lane B) or benzylthiol ( figure 1 , lane C). As a reaction control, 5 μL of buffer instead of catalyst solution was added to the mixture of HES-derivative and peptide ( figure 1 , lane E).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com