Dihydropyrazolohexahydropyridine derivatives, preparation method and application thereof
A technology of dihydropyrazole and hexahydropyridine, which is applied in the field of pyridine derivatives, and achieves the effects of simple process, remarkable practicability and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
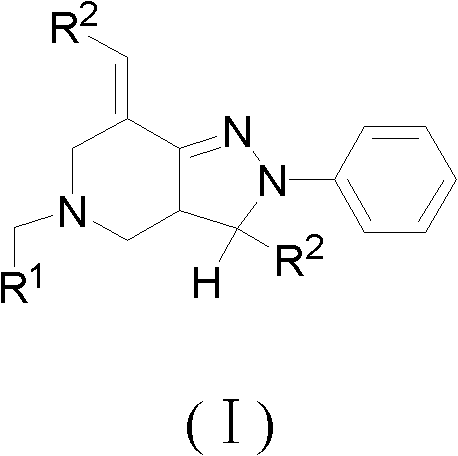
[0046] Example 1: Preparation of 2-phenyl-3-(4-methylphenyl)-5-(4-methylbenzyl)-7-(4-methylbenzylidene)-4,5-dihydro Pyrazolo[4,3-c]hexahydropyridine (Ia);
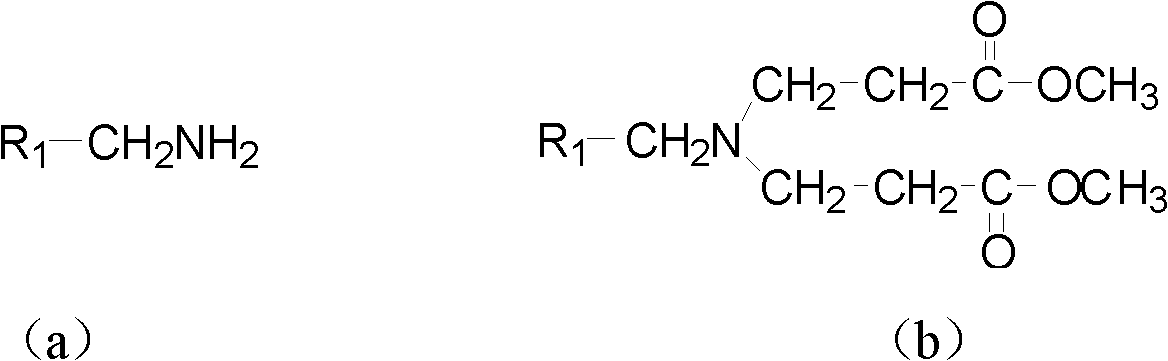
[0047] At room temperature, add 0.16mol methyl acrylate and 7mL methanol to a 100mL three-necked flask, and slowly add a mixture of 0.04mol p-methylbenzylamine and 4mL methanol into the three-necked flask while stirring, so that the temperature of the reaction system does not exceed 50 ℃. After the dropwise addition, heat to reflux for 8 hours. After the reaction is over, reclaim methanol and unreacted methyl acrylate, and distill under reduced pressure to obtain light yellow oily liquid N, N-bis(β-methyl propionate) p-methyl Benzylamine (2a).
[0048]Add 15mL of anhydrous toluene and 0.122mol of sodium metal to a 250mL dry three-necked flask, stir and heat to reflux, add 0.2mL of anhydrous methanol, and then slowly add 0.04mol of N,N-bis(β-propionate methyl ester)) A mixture of p-methylbenzylamine (2a) and 20mL of anhy...
Embodiment 2
[0052] Example 2: Preparation of 2-phenyl-3-(4-methylphenyl)-5-(4-fluorobenzyl)-7-(4-methylbenzylidene)-4,5-dihydropyridine Azolo[4,3-c]hexahydropyridine (Ib);
[0053] Replace p-methylbenzylamine with p-fluorobenzylamine, other raw materials are the same, according to the method of Example 1, obtain (Ib).
[0054] Yield, 77%; mp 190-191°C; 1 HNMR (400MHz,) δ2.35(s, 6H), 2.46(t, J=10.5Hz, 1H), 3.17(m, 2H), 3.33-3.24(m, 1H), 3.58(dd, J=28.3, 13.2Hz, 2H), 4.01(d, J=13.9Hz, 1H), 4.55(d, J=12.5Hz, 1H), 6.81(t, J=7.3Hz, 1H), 6.92(dd, J=12.1, 5.3Hz, 2H), 7.04(dd, J=8.7, 1.0Hz, 2H), 7.11-7.21(m, 10H), 7.25(s, 1H), 7.26-7.30(m, 2H); IR(KBr, cm -1 )3020, 2920, 1710, 1604, 1269, 1201, 1129; Anal.Calcd for C 34 h 32 FN 3 : C, 81.47; H, 6.49; N, 8.34; Found: C, 81.41; H, 6.48; N, 8.38.
Embodiment 3
[0055] Example 3: Preparation of 2-phenyl-3-(4-methoxyphenyl)-5-(4-fluorobenzyl)-7-(4-methoxybenzylidene)-4,5-di Hydropyrazolo[4,3-c]hexahydropyridine (Ic);
[0056] Replace p-methylbenzylamine with p-fluorobenzylamine, replace p-tolualdehyde with p-methoxybenzaldehyde, and other raw materials are the same, according to the experimental method in Example 1, obtain (Ic).
[0057] Yield, 72%; mp 168-169°C; 1 H NMR (400 MHz, CDCl 3 )δ2.43(t, J=10.3Hz, 1H), 3.12-3.25(m, 2H), 3.22-3.34(m, 1H), 3.61(dd, J=38.3, 13.1Hz, 2H), 3.81(d , J=8.3Hz, 6H), 4.03(d, J=13.9Hz, 1H), 4.52(d, J=12.3Hz, 1H), 6.81(t, J=7.2Hz, 1H), 6.83-6.92(m , 4H), 7.05(t, J=8.1Hz, 4H), 7.08-7.23(m, 7H), 7.29(d, J=8.7Hz, 2H); IR(KBr, cm -1 )3066, 2910, 1733, 1612, 1269, 1200, 1121; Anal.Calcd for C 34 h 32 FN 3 o 2 : C, 76.50; H, 6.09; N, 7.84. Found: C, 76.52; H, 6.04; N, 7.87.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com